On October 10, according to foreign media reports, the AstraZeneca/Oxford University Intranasal COVID-19 vaccine did not work well in early studies. The results showed that the vaccine only elicited an antibody response in a “small number of subjects” and that the immune response was weaker than the standard vaccine.
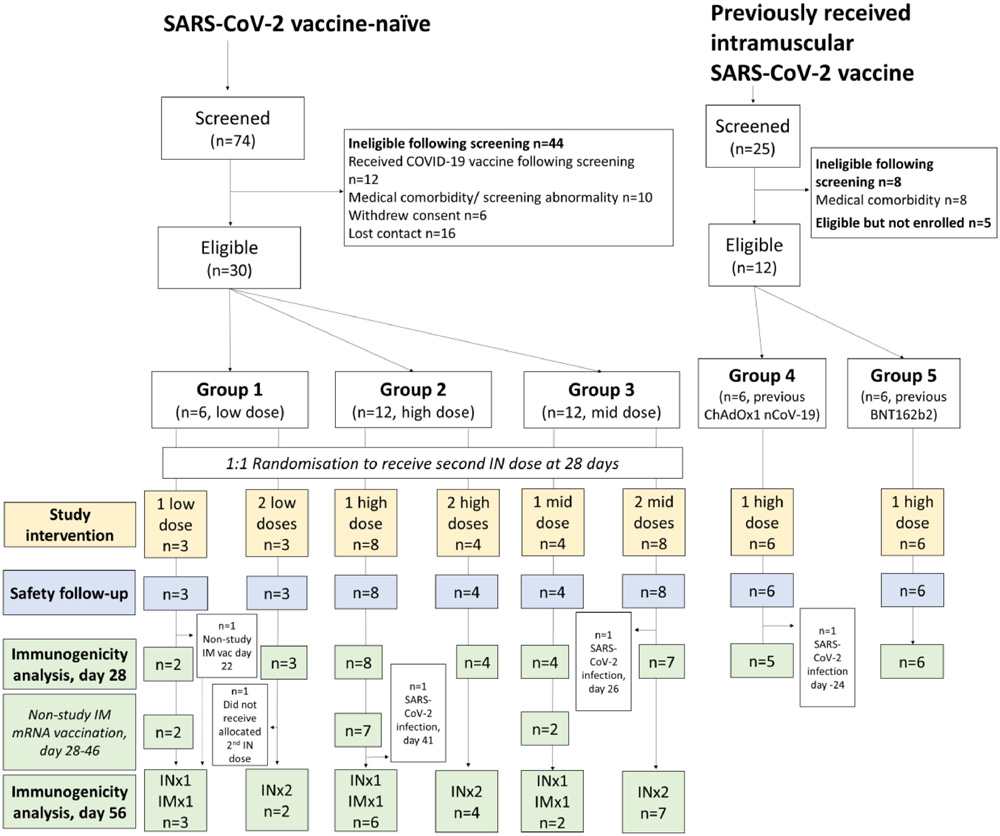
This is a single-centre, open-label, Phase I study conducted in the United Kingdom to evaluate the safety and immunogenicity of intranasal ChAdOx1 nCoV-19 administration in patients with a total of 30 COVID-19 vaccine-naïve subjects. The subjects and 12 subjects who used the Intranasal vaccine as booster shots were enrolled.
The results showed that the vaccine showed acceptable tolerance, but produced a “weak and inconsistent” immune response in subjects, insufficient to support the subsequent development of a Intranasal COVID-19 vaccine.
Previously, Vaxzevria, an adenovirus-vectored COVID-19 vaccine developed by Oxford University and AstraZeneca, was launched in the UK in January 2021. It uses replication-defective simian adenovirus ChAdOx1 as a vector, containing the full-length spike protein of SARS-CoV-2 and a TPA leader sequence. The vaccine will bring AstraZeneca $3.917 billion in sales in 2021.