On October 31, Actinium Pharmaceuticals announced that the pivotal Phase III SIERRA study of its CD45 monoclonal antibody Iomab-B (apamistamab) in patients with relapsed or refractory acute myeloid leukemia (r/r AML) achieved the primary endpoint. In light of this positive result, Actinium plans to submit a Biologics License Application (BLA) to the FDA in 2023 for the treatment of patients with r/r AML over the age of 55.
AML is a myeloid tumor caused by the clonal proliferation of myeloid blasts in peripheral blood, bone marrow or other tissues. The main clinical symptoms include fever, hemorrhage, and anemia, which can lead to complications of infection and hyperuricemia. Most patients with AML will relapse, and patients with r/r AML have a poor prognosis, with a 5-year survival rate of approximately 27%. Multiple studies have shown increased survival in patients undergoing bone marrow transplantation (BMT), however, the vast majority of patients do not have the opportunity to receive BMT because existing therapies fail to alleviate the patient’s disease status or are too toxic.
Iomab-B (initial development code: BC8) is a CD45-targeted radioimmunotherapy developed by the Fred Hutchinson Cancer Research Center, designed to increase patient populations by simultaneously rapidly depleting blood cancer, immune cells, and bone marrow stem cells that specifically express CD45 Opportunity to embrace BMT. In July 2012, Actinium entered into an agreement with the center to acquire global development and commercialization rights for Iomab-B. In April 2022, Immedica Pharma entered into an agreement with Actinium for commercialization rights to Iomab-B in Europe, the Middle East and North Africa. Iomab-B has been granted orphan drug designation by the FDA and the European Medicines Agency (EMA).
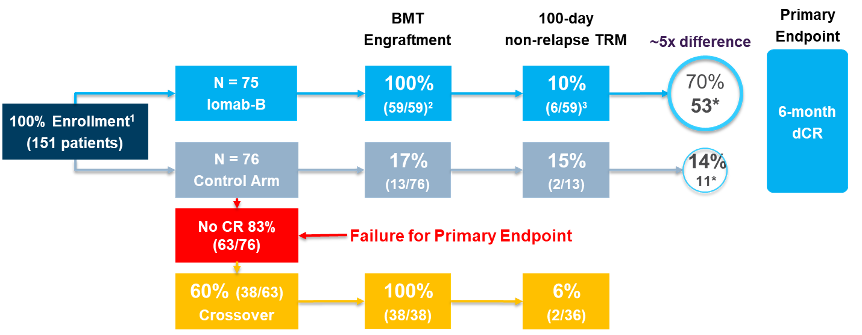
The SIERRA study is a multicenter, randomized, open-label Phase III clinical trial of 153 patients (age ≥55 years) designed to evaluate the treatment activity of Iomab-B versus conventional therapy (leukemia blasts >5% ), efficacy and safety in patients with relapsed or refractory acute myeloid leukemia. Conventional treatment regimens include chemotherapy such as cytarabine and daunorubicin and targeted drugs such as Bcl-2 inhibitors (Venetoclax), FLT3 inhibitors, and IDH 1/2 inhibitors. The primary endpoint of the trial was durable complete response (dCR), and secondary endpoints included overall survival and event-free survival (EFS).
The data showed that patients in the Iomab-B arm achieved a statistically significant 6-month dCR (p<0.0001), and 100% of patients received bone marrow transplantation (BMT), compared to only 18% of patients in the placebo arm. BMT. In addition, Iomab-B was well tolerated, consistent with previous clinical data.
So far, no CD45-targeted therapy has been approved for marketing in the world, only 6 products have entered the clinical stage, and Iomab-B is the only product that has entered the phase III stage.