ALX Oncology’s Evorpacept Receives Fast Track Designation from FDA as First-Line Treatment for Head and Neck Squamous Cell Carcinoma
On August 1, ALX Oncology announced that the CD47 inhibitor evorpacept in combination with pembrolizumab (Keytruda) has been granted FDA Fast Track designation for the first-line treatment of adults with PD-L1-positive advanced head and neck squamous cell carcinoma (HNSCC). patient.
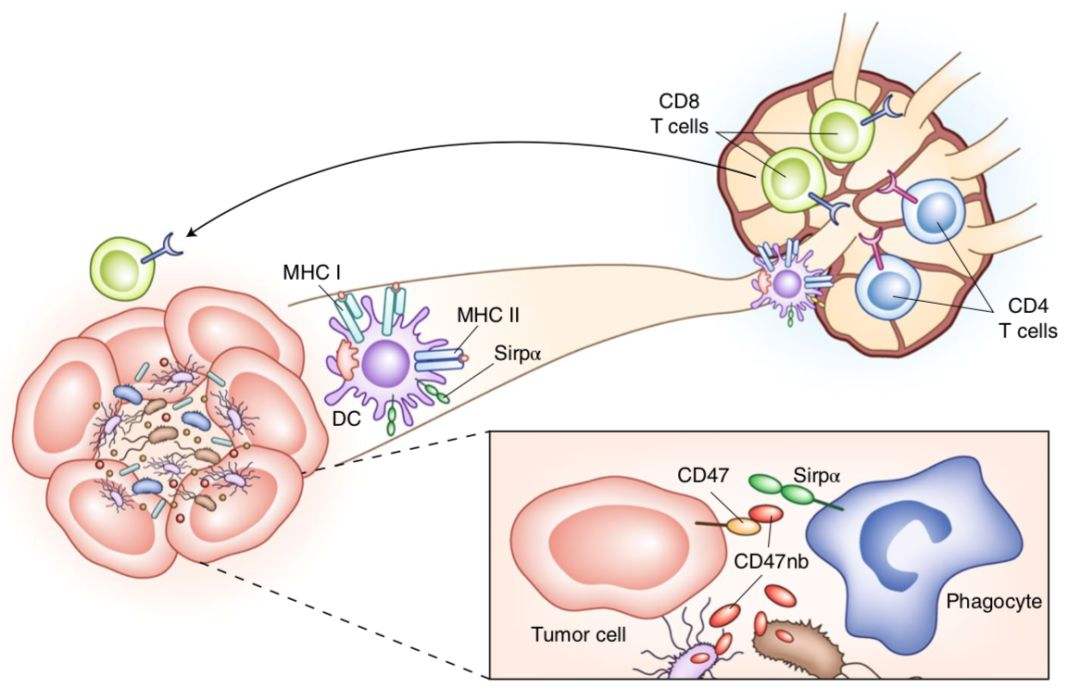
Tumor cells evade the immune system by upregulating the CD47 protein that binds to signal-regulatory protein alpha (SIRPα) on the surface of macrophages to transmit a “don’t eat me” signal. Evorpacept is an Fc fusion protein that targets CD47 and has no Fc activity. The CD47-binding domain of evorpacept is the extracellular domain of SIRPα with high affinity. The specially designed Fc end does not activate Fc receptors, avoids the toxic side effects of macrophages phagocytosing healthy cells expressing CD47, and still maintains a long half-life. The molecular weight of evorpacept is about half that of the antibody, which can enhance its penetration into solid tumors to improve the efficacy.
The FDA’s decision is based on the results of the Phase I ASPEN-01 study. Among patients with advanced HNSCC who had not received checkpoint inhibitor therapy in the second line or above, the objective response rate in the evorpacept plus pembrolizumab group was 40% (n=10). The results show that the evorpacept combination therapy has preliminary anti-tumor activity and a good safety profile, and its efficacy is improved compared to anti-PD-1 monotherapy in a similar population, further supporting the May 2021 collaboration with Merck & Co. Initiated ASPEN-03 study. ASPEN-03 is a randomized, open-label, phase III multicenter clinical trial to investigate the antitumor efficacy of evorpacept in combination with pembrolizumab in first-line treatment of patients with metastatic or unresectable recurrent PD-L1-positive HNSCC.
“The FDA’s grant of Fast Track designation to evorpacept in combination with pembrolizumab for the first-line treatment of HNSCC patients builds on the previously granted Fast Track designation for evorpacept in combination with pembrolizumab in the first-line treatment of HNSCC patients with standard chemotherapy, which highlights the potential of evorpacept in the first-line treatment of HNSCC patients. “The potential clinical benefit in HNSCC, a refractory disease,” said Dr. Sophia Randolph, Chief Medical Officer of ALX Oncology, “We are pleased with the progress of patient enrollment in the Phase II HNSCC program as we seek to advance evorpacept-related studies to help patients. “
CD47 is a popular target in current immune checkpoint inhibitors, and many domestic and foreign pharmaceutical companies have deployed this target.
Magrolimab is a first-in-class monoclonal antibody targeting CD47 obtained by Gilead’s acquisition of tumor immunotherapy company Forty Seven for $4.9 billion in cash in March 2020. In January of this year, magrolimab was suspended by the FDA due to unexpected serious adverse reactions in the clinic; in April, after FDA review, part of the magrolimab clinical trial was resumed.
Recently, AbbVie terminated an early-stage study of its CD47 antibody lemzoparlimab in collaboration with Tianjing Bio for strategic reasons. In September 2020, AbbVie and Tianjing Bio entered into a deal totaling up to $2.96 billion for the development and commercialization of lezolizumab.
In August 2021, Pfizer acquired Trillium Therapeutics for a total of $2.26 billion. Its core products, TTI621 and TTI622, are two SIRPα Fc fusion proteins. In the 2022Q2 quarterly conference call, Pfizer stated that the CD47 program is on schedule and is confident.