On September 15, Jasper Therapeutics announced that its CD117 monoclonal antibody JSP191 has been granted Fast Track designation by the FDA for the treatment of severe combined immunodeficiency patients undergoing allogeneic hematopoietic stem cell transplantation. In 2021, JSP191 was granted orphan drug designation by the FDA for this indication.
Severe combined immunodeficiency (SCID) is a rare and serious genetic disease in which the acquired immune function of patients is significantly weakened or even lost due to defects in immune T cells and B cells that fight infection. Babies with SCID are highly susceptible to a variety of infections after birth and typically require treatment within two years to survive. Existing treatment options include hematopoietic stem cell transplantation, gene therapy, and enzyme therapy.
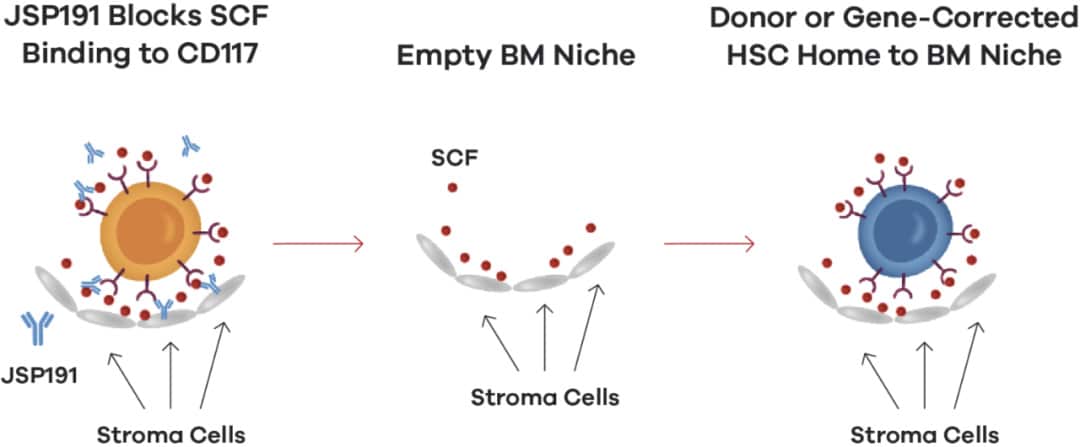
JSP191 is a humanized anti-CD117 (stem cell factor receptor) monoclonal antibody under development. By blocking the signal transduction of stem cell factor receptor, it clears hematopoietic stem cells in the bone marrow and serves as donor or gene-corrected transplant stem cells. Create space. Furthermore, JSP191 does not carry a toxic payload or recruit immune cells, so it is less likely to be off-target.
Currently, JSP191 is being evaluated for efficacy and safety in 4 clinical trials covering acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS), SCID, Fanconi anemia and sickle cell disease. The company also plans to initiate a new study this year exploring JSP191 as a second-line treatment for low-risk MDS.
It is worth mentioning that Amgen originally discovered JSP191 and later awarded its global rights to Jasper Therapeutics in 2019.
“Patients with congenital SCID have severely compromised immune systems and rely on allogeneic hematopoietic stem cell transplantation to create the immune cells needed to fight infection,” said Ronald Martell, president and CEO of the company. poor, cannot tolerate the toxic chemotherapy doses used in transplantation, and may also face severe side effects or transplantation failure. Fast track designation granted by the FDA affirms the potential role of JSP191 in improving clinical benefit in these patients and will allow us Work more closely with the FDA over several months to determine the path to submit a Biologics License Application (BLA).”