Almost all major forms of diabetes are caused by insufficient amounts of beta cells (the cells in the pancreas that produce and distribute insulin). When blood sugar levels in the body rise (as in response to a high-fat diet), beta cells control blood sugar levels by producing and releasing more insulin. But prolonged high blood sugar can impair the ability of beta cells to produce and secrete insulin. This leads to a vicious cycle of ever-increasing glucose levels and declining beta-cell function, culminating in beta-cell death, a phenomenon known as glucotoxicity. Therefore, the preservation and regeneration of β cells is an important goal of diabetes treatment.
Recently, researchers from the Icahn School of Medicine at Mount Sinai have identified a potential therapeutic target for the preservation and regeneration of beta cells, ChREBP. The discovery will help prevent insulin resistance, with major benefits for many people with diabetes around the world. The findings were published in Nature Communications on July 30.
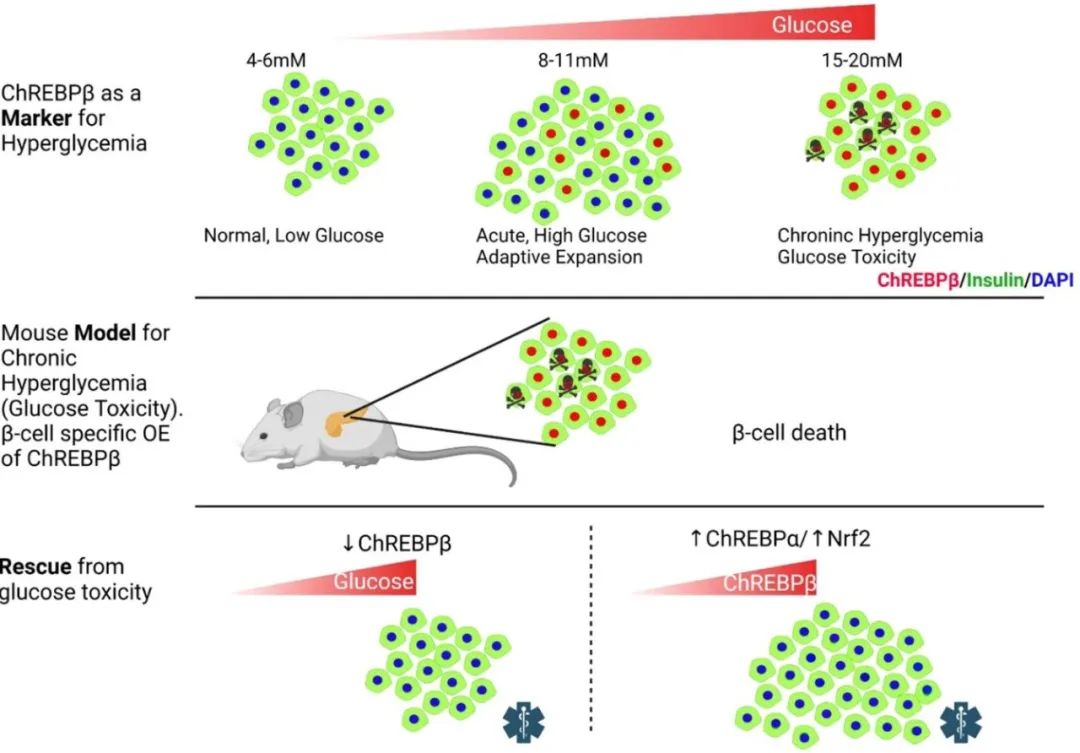
Carbohydrate response element binding protein (ChREBP, gene name MLXIPL) is a glucose-responsive transcription factor that has two major isoforms, ChREBPα and ChREBPβ, both of which are expressed in beta cells. Deletion of ChREBP has been shown to prevent glucotoxicity and glucose-mediated β-cell death. However, the team at the Icahn School of Medicine at Mount Sinai observed that ChREBP is required for glucose-stimulated beta cell proliferation, a key process in the adaptive expansion of beta cells.
Further investigation revealed that chronically increased glucose metabolism can lead to a vicious cycle in which ChREBPβ overproduction leads to glucotoxicity and subsequent death in β cells. In contrast, overexpression of ChREBPα enhanced glucose-stimulated β-cell proliferation, but did not cause β-cell death, as it stimulated the Nrf2 antioxidant pathway, thereby preventing oxidative damage.
Professor Donald Scott concluded: “Traditionally, ChREBP has been thought of as a mediator of glucotoxicity, but we have noticed that this form of ChREBPα appears to protect beta cells. By using a tool we have developed that enables independent detection of these isoforms, we have ChREBPβ was also found to play a key role in the progressive destruction of beta cells. If ChREBPβ is removed or pharmacologically counteracted, the effects of glucotoxicity can be mitigated and beta cells protected.”
The study points to two approaches to balance the effects of ChREBPβ-mediated cell death: 1) overexpression of ChREBPα; and 2) activation of the antioxidant Nrf2 pathway in rodent and human beta cells.
ChREBPβ is required for adaptive β-cell expansion but causes glucotoxicity under conditions of chronic hyperglycemia, and the effects of its overexpression can be rescued by activation of the Nrf2 antioxidant pathway.
Currently, many Nrf2 activators have been or are being tested in clinical trials for diabetes treatment. Bardoxolone methyl (CDDO-Me) was studied for the treatment of chronic kidney disease in diabetic patients, but the clinical trial was terminated early due to cardiovascular safety concerns. Recently, a new phase II clinical trial of CDDO-Me began, which excluded high-risk patients. In addition, some natural compounds that activate Nrf2 are also undergoing clinical trials, although more research is needed to improve their tissue and targeting specificity before the potential of Nrf2-activating compounds to increase or preserve beta cell mass in diabetes is recognized.
Dr. Liora S. Katz said: “Our findings lay the foundation for the preservation of existing beta cell mass and the development of new treatments that have the potential to successfully prevent the progression to insulin dependence in thousands of people with type 2 diabetes.”
Based on these findings, the research team was interested in exploring the effects of ChREBPβ overproduction in patients with type 1 diabetes, because unlike type 2 diabetes, the pancreas of type 1 diabetes does not produce any insulin. In addition, the team plans to investigate whether the vicious cycle observed in this study occurs in other tissues that express ChREBPβ, such as kidney, liver, fat, etc., which may lead to diabetic complications.