On September 10, Bristol-Myers Squibb (BMS) announced that the marketing application of its TYK2 inhibitor deucravacitinib for the treatment of plaque psoriasis has been approved by the FDA under the trade name Sotyktu. Deucravacitinib is the world’s first TYK2 inhibitor approved for marketing, and the National Medical Products Administration also accepted the drug’s marketing application on July 15.
Psoriasis is a widespread, chronic, systemic immune-mediated disease that seriously affects the health, life and work of patients. At least 100 million people worldwide are affected by this disease. Plaque psoriasis is characterized by distinct round or oval patches on the surface of the skin covered with silvery white scales. Existing systemic treatment programs can achieve a certain therapeutic effect, but cannot meet the treatment needs of patients. There are still many patients with moderate to severe psoriasis who are not fully treated or even untreated.
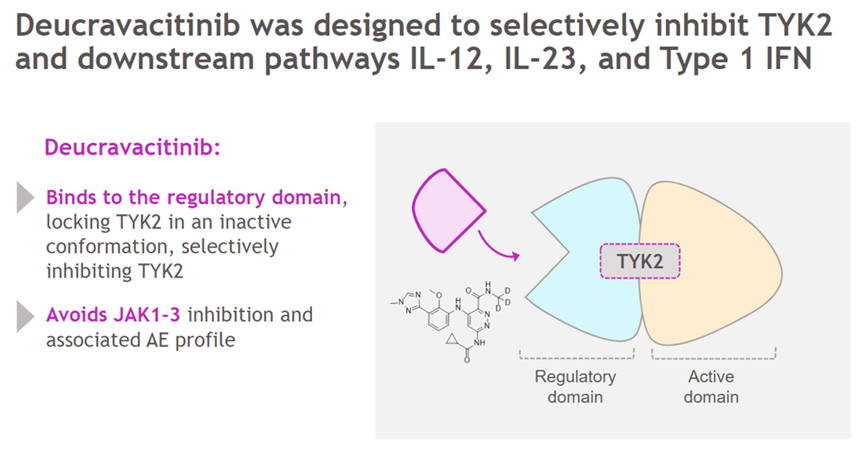
Deucravacitinib is an oral highly selective tyrosine kinase 2 (TYK2) allosteric inhibitor with a unique mechanism of action. It inhibits the signaling of key cytokines (such as IL-23, IL-12, and type I interferons) involved in the pathogenesis of multiple immune-mediated diseases by selectively targeting TYK2. Deucravacitinib achieves high selectivity by binding to the regulatory domain of TYK2, contributing to allosteric inhibition of TYK2 and its downstream functions. Deucravacitinib selectively inhibits TYK2 within the physiological concentration range and does not inhibit JAK1, JAK2 or JAK3 at therapeutic doses.
This FDA approval is based on positive results from two pivotal Phase III clinical trials (POETYK PSO-1 and POETYK PSO-2). Both trials were global, multicenter, double-blind, randomized, placebo, and active drug-controlled studies with 666 and 1020 patients, respectively, and were designed to evaluate deucravacitinib versus apemilast ( apremilast) and placebo in adult patients with moderate-to-severe plaque psoriasis. The co-primary endpoint was the proportion of patients with >75% improvement in the Psoriasis Area and Severity Index (PASI) score and sPGA 0/1 (Static Physician Global Assessment Complete/Nearly Complete Clearance of Skin Symptoms) at Week 16.
The data showed that at week 16, the proportion of patients with a PASI score improvement of more than 75% and sPGA 0/1 was significantly higher in the deucravacitinib group than in the placebo and apremilast groups. The trial also met all secondary endpoints, showing significant and clinically meaningful improvements in measures of symptom burden and quality of life with deucravacitinib. In addition, deucravacitinib was well tolerated with a low rate of discontinuation due to adverse events.
In May of this year, BMS released the data after 2 years of POETYK PSO long-term extension study (POETYK PSO-LTE). The study is an open-label, multicenter Phase III clinical trial of 1452 patients to evaluate the long-term safety and efficacy of deucravacitinib in adults with moderate to severe plaque psoriasis. Completion in July 2026. The data showed that at week 60, 77.7% of patients in the deuterocoxitinib group had a PASI score improvement of more than 75%, and 58.7% of patients had sPGA 0/1.
Dr. Samit Hirawat, Chief Medical Officer of BMS, said: “Today’s approval of Sotyktu is an exciting day for those patients with moderate to severe plaque psoriasis who are dissatisfied with topical and conventional treatments. This is another exciting day for BMS. A remarkable achievement as we propose a novel mechanism of action, the first oral drug approved for the treatment of moderate to severe plaque psoriasis in nearly 10 years. We believe Sotyktu is the treatment for these patients a breakthrough, and we are excited about its potential in other immune-mediated diseases.”
In addition to psoriasis, deucravacitinib is being developed for the treatment of various immune disorders including psoriatic arthritis, scalp psoriasis, systemic lupus erythematosus and inflammatory bowel disease. In addition, for the TYK2 target, BMS has two other drugs (BMS-986202 and BMS-986322) under development. BMS-986202 is in phase II and BMS-986322 is in phase I. It can be said that BMS is a veteran player on the TYK2 inhibitor track.
Global R&D progress of TYK2 inhibitors
Up to now, there are a total of 30 TYK2 inhibitors under research in the world. Except for deuterium cloxitinib, a total of 19 have entered the clinical stage, of which 2 are in the phase III clinical stage and 4 are in the phase II clinical stage.
Brepocitinib
Brepocitinib is a dual TYK2/JAK1 inhibitor first developed by Pfizer. In June 2022, Pfizer and Roivant jointly established Priovant and granted Priovant the global development rights for the product and another TYK2 inhibitor, ropsacitinib, as well as commercialization rights in the United States and Japan, while Pfizer will hold a 25% stake in Priovant. Priovant is currently conducting a registrational Phase III clinical trial of brepocitinib in the treatment of dermatomyositis, with a total of 225 patients enrolled, and is expected to be completed in December 2024.
jaktinib
Jacktinib is a non-selective JAK inhibitor independently developed by Zejing Pharmaceuticals. It has significant inhibitory effects on JAK1, JAK2, JAK3 and TYK2, and has the strongest inhibitory effect on JAK2 and TYK2. In addition, jacktinib can also reduce hepcidin transcription by inhibiting activin receptor 1 (ACVR1) activity, improve iron metabolism imbalance, increase hemoglobin, reduce the incidence of anemia in patients with myelofibrosis and reduce transfusion dependence.
Momelotini
Currently, Jacktinib is in Phase III clinical trials for myelofibrosis, alopecia areata and atopic dermatitis. In addition, in June of this year, Zejing Pharmaceutical announced that the Phase III study of jacktinib in the treatment of myelofibrosis achieved the prespecified primary endpoint in the interim analysis. Based on this, Zejing Pharma will submit a pre-NDA communication application to CDE.
BMS-986202 and BMS-986322
BMS-986202 is a novel TYK2 inhibitor obtained by BMS’s structural modification on the basis of deucravacitinib, and is currently undergoing phase II clinical trials for psoriasis.
NDI-034858
NDI-034858 is an allosteric inhibitor of TYK2 developed by Nimbus and obtained through a structure-based drug design approach. Phase II clinical trials are currently underway for plaque psoriasis and psoriatic arthritis. In a Phase I clinical study in plaque psoriasis, NDI-034858 was shown to improve multiple markers of disease pathology and normalize deregulated molecular and inflammatory pathways in psoriasis. Notably, cell experimental data showed that NDI-034858 was more selective for TYK2 than deucravacitinib.
ropsacitinib
Ropsacitinib is a selective TYK2 inhibitor developed by Pfizer and is currently in Phase II clinical trials for ulcerative colitis, psoriasis and hidradenitis suppurativa.
TLL018
TLL018 is a highly potent and selective oral dual inhibitor of JAK1 (IC50=4nM) and TYK2 (IC50=5nM) developed by Gaoguang Pharma, its potency against JAK2 or JAK3 is greater than 1µM. Phase II clinical trials are currently underway for rheumatoid arthritis and ulcerative colitis.