Ascendis Pharma announced that the FDA has accepted and granted Priority Review status for TransCon PTH for the treatment of adult patients with hypoparathyroidism, with a PDUFA date of April 30, 2023. If approved, TransCon PTH would be the first hormone replacement therapy to treat hypoparathyroidism. TransCon PTH has previously been granted orphan drug designation by the FDA and EMA for the treatment of hypoparathyroidism.
TransCon PTH is a once-daily long-acting parathyroid hormone (PTH) prodrug designed to restore PTH to physiological levels 24 hours a day to address both short-term symptoms and long-term complications of the disease. Currently, TransCon PTH is undergoing Phase III clinical studies in China.
Hypoparathyroidism (HP) is a rare endocrine disorder characterized by insufficient levels of parathyroid hormone (PTH), resulting in low calcium levels and elevated phosphate levels in the blood. Most patients develop disease following injury during thyroid surgery or accidental removal of their parathyroid glands. Conventional treatment with calcium supplements and active vitamin D is not effective in addressing short-term symptoms, long-term complications, or impact on quality of life of hypoparathyroidism. There is currently no replacement therapy to restore physiological hormone levels. As of now, hypoparathyroidism is the last endocrine hormone deficiency disease for which true hormone replacement therapy has not yet been achieved.
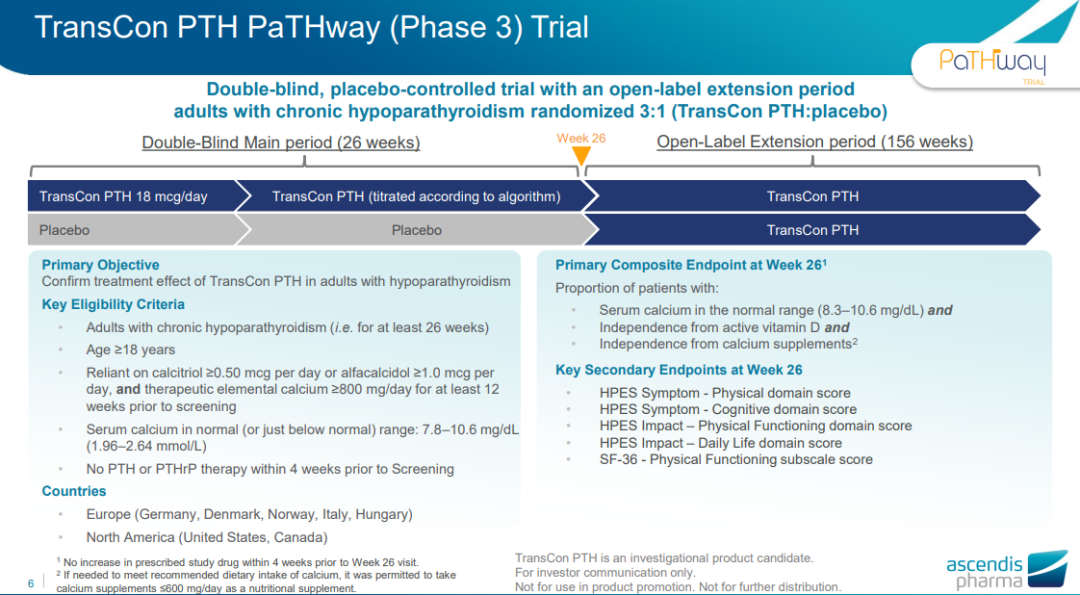
The NDA is based on data from the global Phase III PaTHway trial, the Phase II PaTH Forward trial, and two ongoing open-label extension studies. The PaTHway trial is a double-blind, placebo-controlled Phase III trial to investigate the efficacy of TransCon PTH in adult patients with HP. The primary endpoint of the study was the proportion of patients with normal serum calcium control (8.3 to 10.6 mg/dL) who discontinued conventional therapy (ie, discontinued active vitamin D and calcium ≤600 mg/day) after 26 weeks of treatment; key secondary endpoints It is HP Patient Experience Scale (HPES) symptom-physical score, HPES symptom-cognitive score, HPES impact-physical function scale score, HPES impact-daily life score, and SF-36 physical function scale score.
Results of the study showed statistically significant improvements in the primary composite endpoint and all key secondary endpoints in the TransCon PTH arm compared to the control arm. The primary endpoint was achieved in 78.7% of patients treated with TransCon PTH (48/61) compared to 4.8% in the control group (p<0.0001). Patients achieved statistically significant reductions in both physical and cognitive symptoms as measured by the Hypoparathyroidism Patient Experience Scale (HPES). In terms of safety, TransCon PTH was generally well tolerated with no study drug-related discontinuation events.
Dana Pizzuti, senior vice president and chief medical officer of Ascendis Pharma, said: “We believe that the best way to treat hypoparathyroidism is to restore PTH to physiological levels 24 hours a day. There is currently no such treatment option for U.S. patients, and if TransCon With the approval of PTH, it may become a fundamental therapy for the treatment of hypoparathyroidism.”