In a new study, researchers from Weill Cornell Medical College and Duke University found that antibodies that recruit virus-engulfing white blood cells may protect infants from the potentially severe congenital human cytomegalovirus , HCMV) infection.
Maternal Fc-mediated non-neutralizing antibody responses correlate with protection against congenital human cytomegalovirus infection
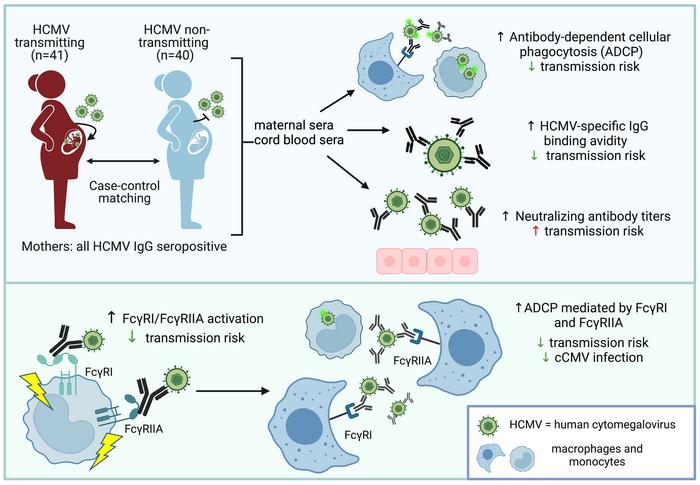
The new study is the most comprehensive analysis of HCMV research to date. The authors examined antibodies in the blood of 81 HCMV-infected mothers, comparing antibodies in mothers who had passed HCMV to their infants (transmission group mothers) and mothers who had not transmitted the virus to their infants (non-transmission group mothers) characteristic. A key finding was that mothers in the transmission group tended to exhibit higher levels of a leukocyte recruitment mechanism against HCMV known as antibody-dependent cellular phagocytosis.
“These findings undoubtedly have implications for the type of immune response that HCMV vaccines should target,” said senior author Sallie Permar, MD, a professor at Weill Cornell Medicine.
HCMV and related viruses in the herpesvirus family are believed to have infected humans and other mammals for at least tens of millions of years. During this time, these viruses have evolved countless tools and strategies to evade the immune defenses of their hosts and establish long-term infections. HCMV is thought to infect most people in developed countries and almost everyone in developing countries. While most infections go unnoticed, HCMV lurks in the body and is often thought to subtly contribute to a variety of diseases in humans, from cancer to heart disease. In addition, a weakened immune system due to the HIV virus, immunosuppressive drugs or very old or very young can also trigger the spread and potentially fatal disease of HCMV.
The survival of HCMV in the human population is partly through mother-to-child transmission during pregnancy. These congenital HCMV infections can lead to stillbirth, hearing loss, abnormal brain development, and other diseases of young children; preventing these diseases is a major public health goal. But traditional vaccine and antibody-based treatment strategies have so far been shown to be ineffective against congenital HCMV infection — highlighting the need to understand how the immune system is effective against the virus.
“Currently, when the mother does have acute CMV infection, or is known to have an infected fetus, we have nothing to offer in terms of vaccines or immunotherapy,” Dr. Permar said.
For the new study, she and her team used cord blood samples from mothers and babies at the Carolinas Blood Bank at Duke University School of Medicine — where Dr. Permar was at the start of the study Carry out research work. Forty-one mothers infected with HCMV passed the virus to their newborns; another 40 did not.
One notable finding concerns “neutralizing antibodies.” These antibodies bind to vulnerable sites in the virus, thereby directly disrupting — neutralizing — the virus’s ability to infect human cells, as well as multiply and spread in standard lab tests. Typically, vaccines against one virus are designed to elicit neutralizing antibodies; the previously unsuccessful HCMV vaccine did the same. But Dr. Permar and colleagues found that higher levels of HCMV-neutralizing antibodies in the mother’s blood were not associated with a lower risk of mother-to-child transmission.
However, they did find evidence that mothers who did not transmit the virus to their babies had higher levels of antibody-dependent cellular phagocytosis. This suggests that this indirect mode of antibody immunity, in which antibody proteins use their “tail” portion, called the Fc region, to recruit virus-engulfing macrophages and other white blood cells, is one that HCMV is not good at evading.
“To combat HCMV, which is good at evading the immune system, we must go beyond the simple concept of neutralizing antibodies and consider antibodies that act in other ways,” Dr. Permar said.
Following the success of the SARS-CoV-2 vaccine, these findings are sure to guide HCMV vaccine efforts at a new pace. Dr. Permar and colleagues are now applying these findings in collaboration with vaccine company Moderna, which is developing a candidate HCMV vaccine using the versatile mRNA platform.