On September 21, 2022, Eli Lilly announced that the U.S. Food and Drug Administration (FDA) has accelerated approval of its precision oncology drug Retevmo (selpercatinib, selpatinib, 40mg and 80mg capsules): for Treatment of patients with RET gene fusion-positive, locally advanced, or metastatic solid tumors that have progressed during or after prior systemic therapy, or have no other satisfactory alternative therapeutic options. This indication was approved under the accelerated approval process based on overall response rate (ORR) and duration of response (DOR) data. Continued approval for this indication will be contingent upon verification and description of clinical benefit in confirmatory clinical trials.
Retevmo is a potent, oral, highly selective, rearrangement during transfection (RET) kinase inhibitor that blocks RET kinase and prevents cancer cell growth. The drug was developed to treat patients with a wide range of cancers with abnormalities in the RET gene.
Notably, Retevmo is the first and only RET inhibitor approved for adult patients with RET fusion-positive advanced or metastatic solid tumors, regardless of tumor type. Tumor-agnostic data from the LIBRETTO-001 trial supporting the approval showed an overall response rate (ORR) of 44% with Retevmo across multiple tumor types.
In addition to the above-mentioned “unlimited cancer” indications, the FDA also converted an accelerated approval of Retevmo to regular approval: for the treatment of locally advanced or metastatic non-small cell tumors that are confirmed by an FDA-approved test product to be positive for RET gene fusions Cell lung cancer (NSCLC) in adult patients. In May 2020, Retevmo received accelerated FDA approval for the treatment of patients with 3 types of tumors (NSCLC, medullary thyroid cancer [MTC], other types of thyroid cancer) with genetic alterations (mutations or fusions) in the RET gene. This routine approval expands the label of Retevmo for the treatment of NSCLC to include patients with locally advanced disease.
“LIBRETTO-001 results demonstrate that selpercatinib is effective in RET-driven cancers, including pancreatic, colon and other demonstrated clinically meaningful and durable efficacy against a variety of tumor types in patients with new treatment options for cancer. These data, along with FDA approval for an unrestricted cancer indication, underscore the need for routine, comprehensive care in patients with multiple tumor types. The importance of genomic testing.”
David Hyman, MD, Chief Medical Officer, Lilly Oncology, said: “Since its initial accelerated approval, Retevmo has transformed the treatment paradigm for cancer patients with RET genetic alterations. Retevmo is the first and only treatment in NSCLC with simultaneous unlimited access to the RET gene. Cancer accelerated and traditionally approved RET inhibitors further support their ability to provide meaningful clinical benefit to patients across different tumor types.”
RET fusions are estimated to be present in approximately 2% of non-small cell lung cancer (NSCLC), 10-20% of papillary thyroid cancer (PTC) and other types of thyroid cancer, and in other cancers (eg, colorectal cancer) subgroups; RET point mutations are present in approximately 60% of medullary thyroid carcinomas (MTCs). RET fusion and RET point mutation cancers are largely dependent on the activation of RET kinases to maintain their proliferation and survival, a dependence often referred to as “oncogene addiction”, making these tumors highly resistant to small molecule inhibitors targeting RET sensitive.
Retevmo is a potent, oral, highly selective, rearrangement during transfection (RET) kinase inhibitor for the treatment of cancer patients with aberrant RET. The RET gene is a proto-oncogene that rearranges during transfection, hence the name, and encodes a cell membrane receptor tyrosine kinase whose abnormality is a rare driver of many types of tumors. Retevmo is designed to inhibit native RET signaling as well as expected mechanisms of acquired resistance and is being developed to treat patients whose tumors harbor aberrant RET kinases.
The above-mentioned accelerated approval for “all cancers” and conventional approval for NSCLC are supported by data from the pivotal Phase 1/2 LIBRETTO-001 trial. The trial is the largest clinical trial using a RET inhibitor to treat patients with RET-driven cancers. This multicenter, open-label, multicohort study included patients with locally advanced or metastatic RET-driven solid tumors, including NSCLC. The primary efficacy outcomes were ORR and DOR, as assessed by a blinded independent review committee (BIRC). Prespecified secondary endpoints included central nervous system (CNS) ORR and CNS DOR.
– RET fusion-positive solid tumors: Among the 41 patients in the “cancer-free” dataset, the most common cancers were pancreatic cancer (27%), colorectal cancer (24%), salivary gland cancer (10%) ) and unknown primary cancer (7%). Thirty-seven patients (90%) had received prior systemic therapy (median: 2 [range 0-9]; 32% received 3 or more). The efficacy results are summarized as follows:
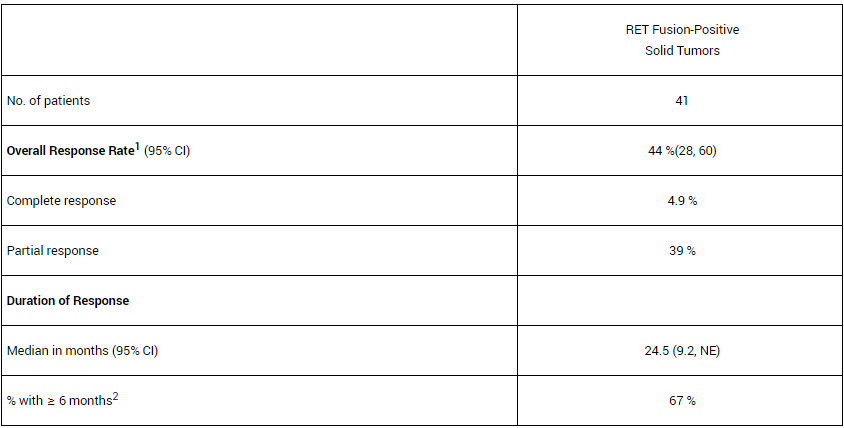
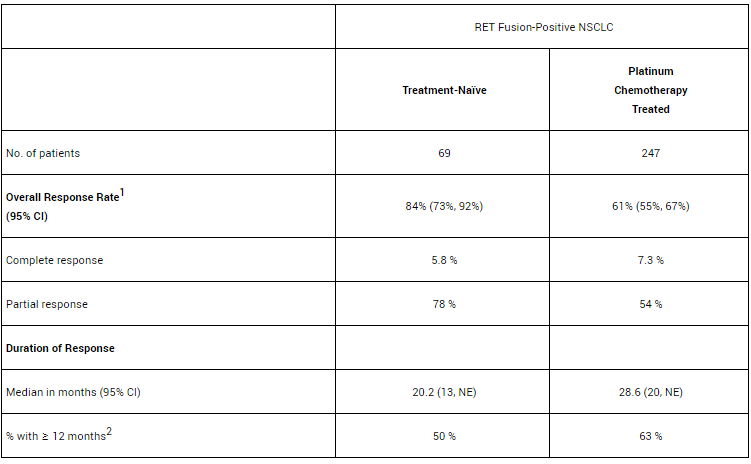
– RET fusion-positive NSCLC: The efficacy results of patients with platinum-based chemotherapy and treatment-naive RET fusion-positive NSCLC are summarized below:
Efficacy results of CNS metastases RET fusion-positive NSCLC patients: (1) Among patients with CNS metastases RET fusion-positive NSCLC who received prior treatment, the ORR of intracranial lesions was 87.5% (n=14/16); 39% of responders had intracranial DOR ≥12 months. (2) Among the newly diagnosed RET fusion-positive NSCLC patients with CNS metastases, the ORR of intracranial lesions was 80% (n=4/5); 38% of the remission patients had intracranial DOR ≥ 12 months.
In the LIBRETTO-001 safety evaluation population (n=796), the most common adverse reactions (≥25%) in patients with advanced solid tumors were edema, diarrhea, fatigue, dry mouth, hypertension, abdominal pain, constipation, rash, nausea, and Headache. The most common grade 3 or 4 laboratory abnormalities (≥5%) were lymphopenia, increased alanine aminotransferase (ALT), increased aspartate aminotransferase, decreased sodium, and decreased calcium.
References:
FDA Approves Lilly’s Retevmo® (selpercatinib), the First and Only RET Inhibitor for Adults with Advanced or Metastatic Solid Tumors with a RET Gene Fusion, Regardless of Type









