The protein component of the biological product from the production cell line itself is called host cell protein (HCP). HCPs are complex in composition, including both cell-secreted and structural proteins. Due to the wide variety, the physicochemical properties of these proteins, such as isoelectric point, hydrophobicity, and relative molecular mass, are often significantly different. HCP may affect the quality of product use, such as:
1. It may cause an immune response or allergic reaction in the patient;
2. It can cause aggregation or fragmentation of biological products;
3. Promote the degradation of polysorbate, resulting in visible foreign matter containing degradation products.
Therefore, the content of HCP residues in biological products is usually regarded as a critical quality attribute (CQA) of a product, an important evaluation index for process robustness monitoring, and an important quality control index for products.
Regulations
In the US, EU and national regulations, HCP is defined as a process-related impurity. Guidance states that it should be minimized through the use of appropriate, controlled manufacturing processes. ICH Q6B states that manufacturers of biologics should evaluate possible impurities and develop specifications for impurities based on preclinical data and clinical experience. Among the residue limits of final products, the recommended limits of China and the United States are all below 0.01%.
HCP ELISA assay method
Enzyme-linked immunosorbent assay (ELISA) is a method recommended by the pharmacopoeia of various countries to detect residual HCP in biological products. This method has the characteristics of simple operation, rapidity and high throughput. In USP and EP, the host cell protein detection method is described in detail from the aspects of antigen-antibody preparation, method development, antibody characterization and method validation.
3.1 Development Cycle
The common HCP ELISA assay method development plan should be determined according to the different stages of the project. Commercially available HCP kits are selected during product development, and this assay can usually be used until clinical phase II (Figure 1A). In Phase III and beyond, the platform process HCP method or the upstream process exclusive HCP method should be preferred. If you want to apply the commercial kit to Phase III and post-marketing, it should be fully demonstrated that the main reagents (such as standards, antibodies, etc.) in the kit are suitable for HCP detection in this process. Considering that the kits come from external suppliers, and the biological product manufacturer has less control over them, when the key reagents (such as antibodies) are replaced, the method consistency before and after the replacement should be demonstrated.
The platform process HCP method refers to the detection method developed using the company’s specific host cell line, HCP standards and corresponding antibodies produced according to the upstream platform process. The same method can be used for HCP detection when an item has similar upstream conditions. Figure 1B shows that if an HCP method based on a platform process already exists at the time of product development, this method can be used in all stages of product development. If the cell culture process changes significantly from the platform process, and potentially introduces a significantly different HCP population, a switch to a proprietary HCP approach is required prior to Phase III/process validation.
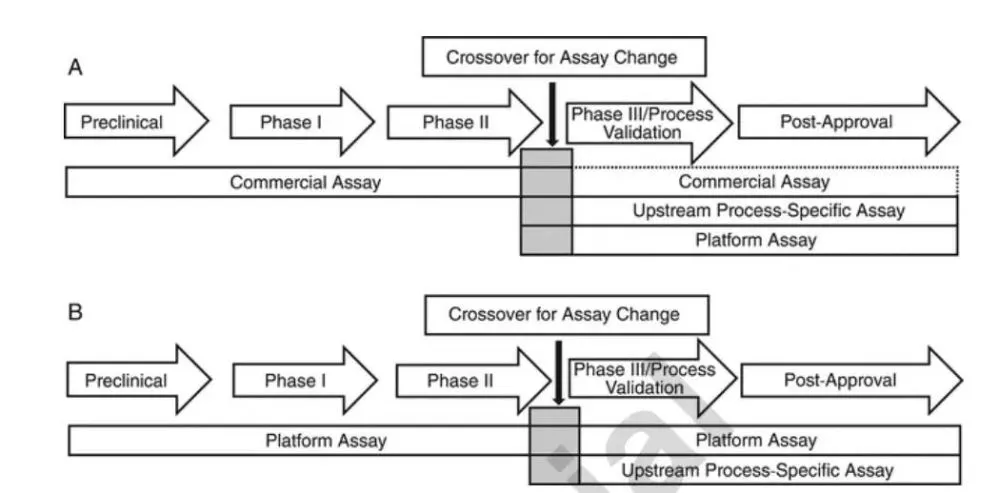
Selection strategy of HCP analysis methods in different cycles of product development
A: Select commercial kits during product development
B: Choose a platform HCP analysis method during product development
3.2 HCP antigen preparation
3.2.1. Empty cell culture
The production cell line transfected with the empty plasmid was used to generate HCP under cell culture conditions close to the production process. The benefit of generally using cell pools rather than monoclonal cultures is that more variability can yield more HCPs.
Since changes in cell viability, metabolism, and density can all alter the HCP profile of the antigen, the cell culture can be modified slightly to generate different HCP combinations (e.g., allowing longer fermentation times, artificially changing culture conditions, reducing cell viability, etc.) , to obtain a broader HCP spectrum.
HCP antigens can be generated from representative small scale, pilot scale or production scale. A pilot or production scale can best simulate the actual process. However, if only one large-scale production is applied, it may not reflect the normal variation in the cell culture process; instead, several small-scale productions can be combined to better reflect the variability caused by different cell culture processes.
3.2.2. HCP harvest
HCP antigens can be prepared from cell lysates, HCCF, or a mixture of both, as shown in Figure 2. If the antigen is prepared from HCCF, harvesting of cells can be delayed to allow more cells to lyse and release HCP, thereby broadening the HCP spectrum. After the upstream fermentation process, the resulting HCP antigen is subjected to minimal processing to reduce HCP loss. The same or similar HCCF step as the actual downstream production process is usually chosen to remove cellular debris, and then the HCCF is processed by ultrafiltration/diafiltration, buffer exchange (e.g. PBS or HEPE) and concentration. The loss of HCP to the filtration membrane should be minimized, eg, use a filter with a molecular weight cut-off of 10 kDa or less.
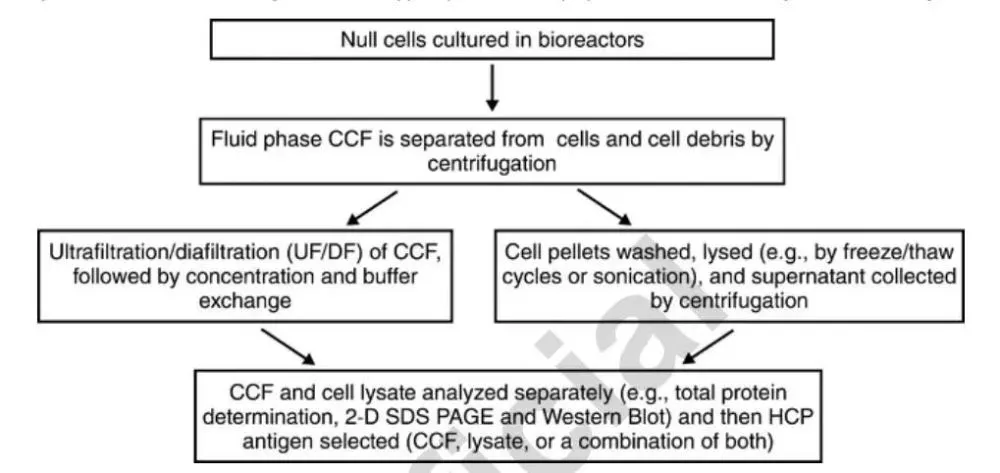
Typical process of mammalian HCP antigen preparation
3.2.3. HCP quality analysis
Analysis of HCP quality should be performed prior to animal immunization:
1. The total amount of antigen prepared;
2. Confirmation of HCP antigen without any product contamination by immunoassay or mass spectrometry;
3. Determine the presence of as much HCP protein as possible by 1-D or 2-D polyacrylamide gel electrophoresis.
The HCP antigen is also used as a standard, so also ensure:
1. The production volume should be large enough to provide multi-year (usually 10-20 years) inventory;
2. The antigen preparation process should be recorded in detail so as to facilitate the retrospection of later reproduction;
3. The antigenic composition of HCP should also be comprehensive enough to tolerate process manufacturing changes during the product life cycle;
4. HCP standards should be monitored for stability to avoid degradation.
3.2.4. Preparation of anti-HCP antibodies
Since the anti-HCP antibodies produced may have a long lifespan (e.g., the expected life cycle of the product or platform), as many antibodies should be produced as possible. Since CHO is a mammalian cell, the use of species further away from mammals in the phylogenetic tree (such as chicken) can help to generate antibodies against mammalian conserved proteins and help improve antibody coverage. Prior to immunization, it should be confirmed whether animals have pre-immune antibodies to the product drug, and animals with positive reactions should be excluded.
Each animal can be boosted multiple times to produce high-titer, high-affinity antibodies. Low molecular weight HCP antigens are often poorly immunogenic, and low molecular weight HCP antigens can also be collected separately, and then different immunization strategies can be implemented to enhance the immune effect.
Serum from each individual should be subjected to titer or Western blot analysis prior to pooling of antisera to screen and remove low titer, immunoreactive only partial HCP, and nonspecific binding antibodies. The resulting antibody library should also demonstrate HCP coverage and specificity.
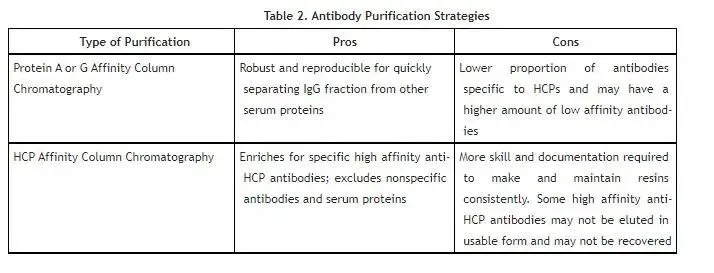
Advantages and disadvantages of different purification methods
3.3 Coverage study of HCP antibodies
There are two methods for assessing coverage: two-dimensional electrophoresis combined with western blotting (2-D Western blot) or immunoaffinity capture. USP
Two methods of antibody coverage assessment were compared (Figure 4).
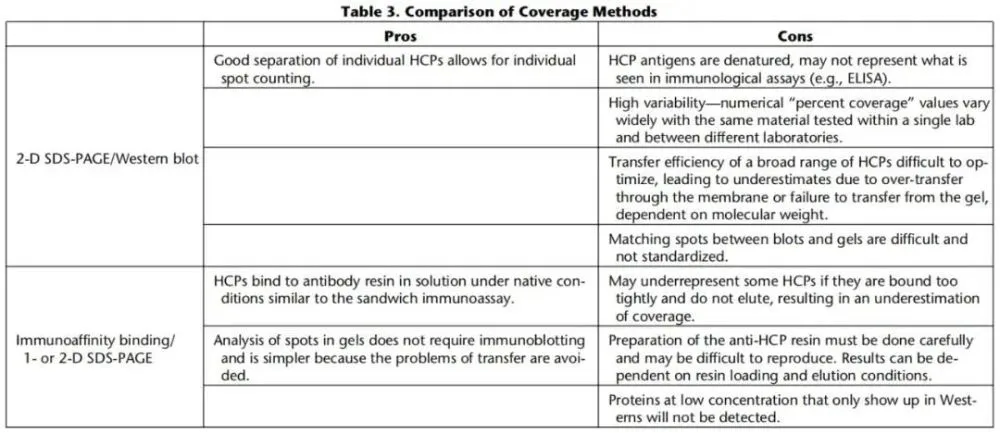
3.3.1. 2-D Western blot
Two-dimensional electrophoresis was performed on the same sample using two identical gels, one of which was developed by Western blot, and the other was stained with silver/fluorescence; the protein spots in the two gels were then matched and analyzed by software, and the coverage level was calculated (Fig. 5). This method is simple to operate, but has poor repeatability and difficult data comparison and analysis; and the HCP in the method is under denaturing conditions, which is different from the natural state of HCP in ELISA detection (Figure 6).
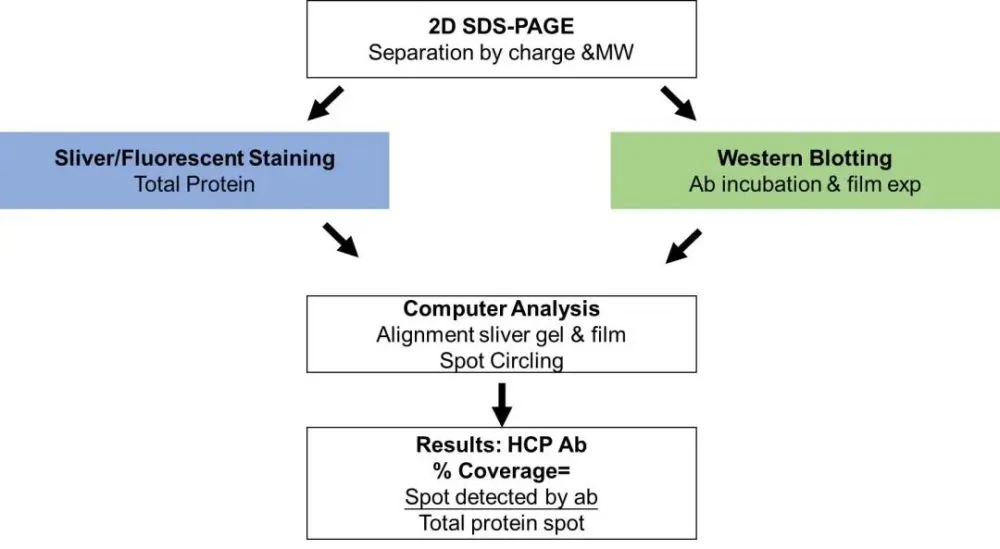

Coverage comparison chart
Left: 2-D SDS-PAGE fluorescent dye staining of CHO HCP
Right panel: Western blot analysis of the same sample as the left panel
3.3.2. Immunoaffinity 2D electrophoresis
Immunoaffinity 2D electrophoresis is a method based on immunochromatographic columns and 2D electrophoresis. First, the HCP antibody is coupled to the chromatography medium, and then the HCP sample is loaded. During the process, the HCP antibody can capture the recognized HCP, while the unrecognized HCP will flow through, and finally the HCP is enriched by elution. The pre- and post-capture HCPs were subjected to two-dimensional electrophoresis silver staining analysis, respectively, and the antibody coverage was calculated (Figure 7).
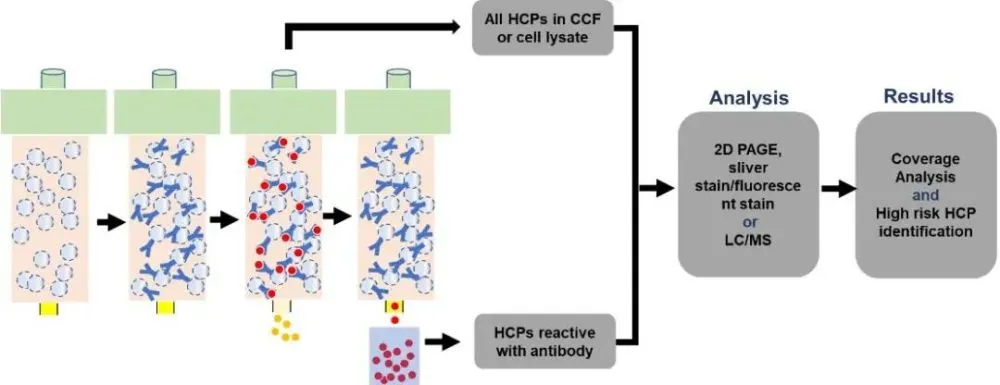
3.3.3. Immunoaffinity-liquid phase mass spectrometry (LC-MS/MS) analysis
On the basis of immunoaffinity, coverage analysis can also be performed using LC-MS/MS, and HCP can be quantitatively analyzed. The LC-MS/MS method has several advantages:
1. Potential high-risk HCPs can be identified early in product development;
2. Relative levels of individual HCPs can be determined. However, the sensitivity of this method presents a very high challenge due to the very low levels of individual HCPs in the final product.
Validation of HCP ELISA assay method
Since there are thousands of HCP antigens and corresponding antibodies, and the types and quantities of HCPs decrease with the purification steps, this poses a challenge to the validation of HCP immunoassay methods. For validation of intermediate process samples, the focus is on method accuracy (spike recovery), dilution linearity, and precision. The final product should be inspected from the aspects of accuracy, precision, dilution linearity, specificity, limit of quantification and so on. The issues that need to be considered in the validation of ELISA methods are discussed below.
4.1 Limit of Quantitation
ELISA limits of quantification are often at ng/mL levels. First add 10 ng/mL of HCP standard to a solution of serially diluted DS. The recovery rate of standard addition can be set to 70%-130% (the recovery rate of standard addition at the limit of quantification can be set to 50%-200%), and calculate the minimum dilution ratio of DS that meets the requirements. The second step is to add HCP standards of different concentrations at the minimum dilution ratio, and the recovery rate should be 70%-130% (the recovery rate of standard addition at the limit of quantification can be set to 50%-150%), which meets the minimum requirements above. The concentration is the limit of quantification. The results are usually satisfactory for at least three experiments performed by different people and on different days.
4.2 Sample dilution linearity
In the HCP immunoassay, due to a certain amount of antibodies coated in the 96-well plate, some HCP content may exceed the amount of HCP antibody, and the data will be distorted when the HCP antibody is saturated; and the no-wash ELISA detection method is in high At high concentrations of HCP, there is a Hook effect. Therefore the amount of HCP in the reaction should be lower than the amount of antibody. In some cases, the method has limited sensitivity and no linearity is observed even when the sample is serially diluted to the limit of quantitation. In this case, the highest HCP value within the validated range should be reported. Non-linearity in dilution results is not uncommon and is generally considered to be an insufficient amount of antibody, but there are other potential causes such as antibody-product reaction or matrix interference, presence of non-specific antibodies, and HCP aggregation in the product.
Other commonly used HCP detection, quantification and characterization analysis methods
HCP can be detected in a variety of ways. In addition to the most common ELISA, other commonly used electrophoresis methods include 1-D SDS-PAGE, 2-D SDS-PAGE and CE-SDS, etc.; commonly used immunoblotting methods include 1-D/2-D Western blot; chromatography methods There are reversed-phase chromatography and proteomic methods.
As knowledge of the HCP species in products grows, specific protein risk assessments can be performed and, where necessary, testing for specific HCP impurities. For example, developing quantitative HPLC-MS/MS methods to quantify peptide fragments of specific HCPs.
discuss
Due to the heterogeneity of HCPs, not every HCP can produce antibodies in animals. HCPs with high immunogenicity produce more antibodies and usually dominate the ELISA signal, while HCPs with low immunogenicity do not have enough antibodies. antibodies to recognize them. The HCP detection readout is therefore an “immunity-weighted” of all HCPs. Nonetheless, ELISA is capable of measuring the broadest spectrum of HCPs and is the most commonly used HCP assay in the industry. As a complement, traditional HCP detection methods such as 1-D/2-D SDS-PAGE remain useful tools for HCP characterization. Due to the complexity of HCP detection, appropriate detection methods need to be selected according to the product life cycle. Developers should conduct sufficient characterization studies, conduct necessary method validation, meet regulatory requirements, and ensure patient safety.
References
[1] USP, “Residual Host Cell Protein Measurement in Biopharmaceuticals”.
[2] EP 2.6.34, “HOST-CELL PROTEIN ASSAYS”.
[3] ICH Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products
[4] Wang X, Host cell proteins in biologics development: Identification, quantitation and risk assessment. Biotechnology and Bioengineering. 2009; 103:446–458. doi: 10.1002/bit.22304.
[5] Jones M, “High-risk” host cell proteins (HCPs): A multi-company collaborative view. Biotechnology and Bioengineering. 2021;118:2870–2885. doi: 10.1002/bit.27808.
[6] Dixit N, Residual Host Cell Protein Promotes Polysorbate 20 Degradation in a Sulfatase Drug Product Leading to Free Fatty Acid Particles. J Pharm Sci (2016) 105:1657–66. doi: 10.1016/j.xphs.2016.02.029
[7] Cui Xinling. Research progress on host cell proteins of genetically engineered drugs [J]. Journal of Pharmaceutical Analysis, 2019(9):9.









