On October 24, Alpine Immune Sciences announced that it had terminated two clinical trials of the conditional CD28 costimulator and dual PD-L1/CTLA-4 checkpoint inhibitor davoceticept (ALPN-202) due to a second patient death .
The core technology of Alpine Immune lies in the targeted engineering of immunoglobulin superfamily (IgD) and TNF superfamily proteins (TD). At present, the Alpine Immune pipeline mainly deploys three drugs, namely ALPN-101, which is modified for ICOSL IgD, ALPN202, which is modified for CD80 IgD, and ALPN-303, which is modified for TNF superfamily receptor protein TCAI.
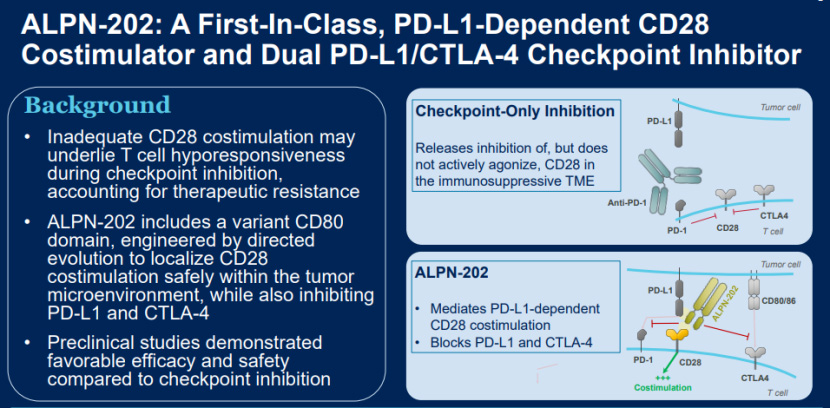
Davoceticept (ALPN-202) is a recombinant protein that has undergone directed evolution of the antibody variable region fragment of CD80 to give it the ability to bind to PD-L1, and then fused this protein with the antibody Fc fragment to form a recombinant protein. On the one hand, this recombinant protein can activate the CD28 receptor when it binds to PD-L1, on the other hand, it can block the binding of PD-L1 to PD-1, and inhibit the binding of CD80/86 to CTLA-4.
For ALPN-202, Alpine has conducted two Phase I clinical trials, NEON-1 and NEON-2. NEON-1 is an open-label, dose-escalation and expansion Phase I clinical study designed to evaluate the efficacy and safety of ALPN-202 as a monotherapy in adult patients with advanced malignancies.
At the 2021 ASCO annual meeting, Alpine announced the research data of NEON-1. The results showed that ALPN-202 treatment resulted in 60% disease control in patients, and no safety cases of cytokine risk were found. ALPN-202 was well tolerated with dose-dependent PK/PD. Early clinical benefit has been found in cancers that do not respond to conventional immunotherapy.
In June 2021, Alpine and Merck signed a collaboration agreement to evaluate the efficacy and safety of the conditional CD28 costimulatory and dual-checkpoint inhibitor ALPN-202 in combination with Merck’s anti-PD-1 therapy Keytruda in the treatment of cancer.
NEON-2 is a dose-escalation and expansion phase I clinical study evaluating ALPN-202 in combination with Merck’s Keytruda in patients with choroidal melanoma. On March 9, 2022, a patient in the NEON-2 study died of cardiogenic shock, and the FDA partially suspended the clinical trial. Recently, the NEON-2 study saw the death of a second patient with metastatic colorectal cancer who had previously undergone colectomy and multiple systemic chemotherapy. Based on the above results, Alpine decided to terminate the ALPN-202 clinical study.
Dr. Mitchell H. Gold, Executive Chairman and Chief Executive Officer of Alpine, said: “Patient safety remains the company’s top priority. We have identified the termination of patient recruitment for the ALPN-202 clinical trial, and we will continue to work with the FDA and Merck’s Research Safety Monitoring Committee. Working with researchers to further investigate this serious safety issue.”
In the future, Alpine will shift its R&D focus to the development of BAFF/APRIL antagonist ALPN-303 for the study of autoimmune diseases. Both the Phase I RUBY-1 trial of ALPN-303 in healthy volunteers, as well as preclinical studies, showed that the drug significantly improved efficacy against BAF. Data from the Phase II RUBY trial evaluating ALPN-303 in systemic lupus erythematosus are expected in 2026.
In addition, a phase II clinical trial of a CD28/ICOS co-stimulatory antagonist jointly developed by Alpine and AbbVie for the treatment of systemic lupus erythematosus is also underway.