In order to carry out anti-cancer metastasis, it is necessary to understand the metastasis route of cancer cells, the process of metastasis and the steps of metastasis. Metastasis of cancer cells is a very complex and dynamic continuous biological process, which consists of several relatively independent steps.
In order to explain the convenience of understanding, the author tries to summarize the biological characteristics and biological behavior of metastasis into eight steps to describe. However, cancer cell metastasis is a continuous and dynamic process, and the steps cannot be clearly divided, but only for the convenience of description and understanding, as well as the arrangement of the intervention and interception of each metastasis step, and the design of prevention and control strategies.
1. Separation of cancer cells from the primary tumor
The first step in metastasis is the separation and shedding of cancer cells from the primary tumor. Why do cancer cells detach from the primary tumor? Is it necessity or chance? How do cancer cells detach from the primary tumor? This starts with an understanding of cell adhesion and metastasis.
Cell adhesion and metastasis: The adhesion between cancer cells and cancer cells is lower than that of normal tissues. When cancer cells are separated from the parent tumor, the adhesion between cancer cells and cancer cells must be reduced. The reasons are:
1) The increase of negative charge density on the surface of cancer cells increases the electrostatic repulsion between cells, which may make tumor cells easy to fall off into a free state in tumor tissue.
2) The more important reason is that, from a molecular point of view, there are a group of molecules that regulate cell-cell adhesion between cancer cells – cadherin family molecules, tumor cell adhesion molecules (cell abhesion molecule, CAM) ) expression decreased, which reduced the adhesion of cancer cells and allowed them to detach from the primary tumor.
3) The increased intercellular pressure may promote the separation and shedding of cancer cells from the primary tumor.
Therefore, the separation and shedding of cancer cells from the parent tumor is the key to cancer cell metastasis. If no cancer cells are shed from the parent tumor, there is no possibility of metastasis. Why do cancer cells separate and fall off? Is it a cell membrane problem or a cell nucleus problem? How can I manage to keep it from falling off? It needs to be further studied and explored from the molecular level and the gene level.
2. Cancer cells separated from the parent tumor first adhere to the cell matrix
The second step in metastasis is that the detached cancer cells from the parent tumor must first adhere to the cellular matrix. The interaction between cells and the extracellular matrix (ECM) plays an important role in tumor invasion. The basement membrane (BM) is formed in the extracellular matrix beneath epithelial and endothelial cells, and cancer cells must first come into contact with it before it can degrade and pass through the ECM. To enter the blood circulation, shed cancer cells must secrete enzymes to degrade and cross the ECM barrier. The ECM is mainly composed of four molecules: collagen, elastin, glycoprotein and proteoglycan. The degradation of these structures is conducive to the invasion and metastasis of cancer cells.
It has been found that the invasion and metastasis of cancer cells are positively correlated with a variety of degrading enzymes. Including matrix metalloproteinase (MMP), serine protease (including urokinase-type and tissue-type plasminogen activator, cysteine protease and aspartic protease group, etc.).
Among them, MMP and urokinase-type plasminogen activator (uPA) and its receptor (uPAR) system are the most widely studied.
Since the above two proteases play an important role in ECM degradation and are closely related to cancer metastasis, many researchers did not intend to inhibit tumor metastasis by inhibiting the activity of proteases.
3. The movement of cancer cells
The third step in metastasis is the movement of cancer cells. For the metastasis of cancer cells to migrate from site A to site B, cancer cells must have the ability to move. If there is no movement of cancer cells, there is no possibility of cancer metastasis. The mechanism of metastasis and migration of cancer cells is initiated by motor factors, and after the combination of motor factors and receptors, the movement of cancer cells is triggered by information transduction. Cancer cell motility is necessary for cancer cells to invade surrounding tissues and invade and exit blood vessels. Why do shed and separated cancer cells still move? How does it move? Cancer cells themselves have amoebic movement. As early as 1863, pathology Virchow proposed that tumor cells have amoebic movement, which has been confirmed by many studies. The movement of cancer cells is a continuous process, and the movement of cancer cells is similar to that of white blood cells, mainly manifested in pseudopodia-like stretching. During its movement, pseudopodia are first formed and adhered to them by receptors on the corresponding basement membrane, pulling the part of the cell body forward, and then after the cell body contracts, the cell releases the adhesion receptors, making the entire cell move. This is a repetitive and coordinated process, and many mechanisms remain unclear.
Adhesion plays a dual role in the invasion and movement of cancer cells: on the one hand, cancer cells must be detached from the primary tumor where they originally adhered before they can invade, and on the other hand, cancer cells need to adhere to move. Cancer cells obtain the impetus to move from the continuous adhesion contact and adhesion release, so the process of cancer cell invasion and movement is the process of adhesion. Therefore, the relationship between CAM, adhesion and cancer metastasis is a hot spot in the study of cancer invasion and metastasis. If it can inhibit cell movement. It may become one of the ways to block tumor metastasis.
4. Drill into and pass through the vessel wall
If cancer cells cannot invade and pass through the walls of microvessels, there is no possibility of cancer cell blood metastases, and cancer cells will not metastasize to distant organs through blood flow. If cancer cells can be prevented from penetrating the blood vessel wall, they can “keep the enemy out of the country” and prevent them from entering the blood circulation, which may become one of the ways to block cancer metastasis. After cancer cells pass through the extracellular matrix (ECM) and reach the blood vessels, they can secrete enzymes to degrade the ECM through the same mechanism as above, and then pass through the basement membrane outside the blood vessels. “Amoeba”-like movement, burrows into the vascular endothelial cell space and through the vascular wall, into the blood circulation.
5. Cancer cells run in the blood
The fifth step in metastasis is the movement of cancer cells in the bloodstream. Cancer cells pass through the blood vessel wall and enter the venous blood flow, and float and transport with the blood flow in the blood circulation. Cancer cells in the blood circulation may be a single cancer cell or a group of cancer cells or form a tumor thrombus. Factors that cannot adapt to it – for example, some cancer cells lack deformability, so that they cannot penetrate through the blood vessel wall, or cancer cells lack the ability to form tumor thrombi, or lack adhesion molecules on the surface of cancer cells. Or due to the inability to adapt to environmental factors from the host – such as the body’s immune system, turbulent blood flow, vertical pressure on capillaries, non-specific killing of NO produced by endothelial cells, etc., the vast majority of cancer cells in the circulation are killed. In the end, no metastases can be formed.
Cancer cells are phagocytosed in the blood circulation by NK cells, LAK cells, T cells, phagocytes, cytokines, INF, TNF, and gene nm23, which are inherent in the human body against cancer cell lines. kill. If cancer cells and platelets, leukocytes, fibrin deposits, etc. aggregate with each other to form tiny tumor thrombi, it will help protect cancer cells from mechanical operations such as blood flow shock and phagocytic damage by immune cells and stagnate and adhere to capillaries wall, thereby increasing the transfer power.
6. Cancer cells penetrate the blood vessel wall again
This is a dangerous stage of metastasis. After the cancer cells penetrate the blood vessels, they grow locally and form a small tumor clone around the blood vessels, which is called micrometastases. How does it penetrate the blood vessel wall? Cancer cells with high metastatic potential in the blood circulation can attach to the inner wall of the microvessels while running with the blood, and some of them can pass through the base of the inner wall of the blood vessels, and then go out of the blood vessels. Why do cancer cells burrow out of the blood vessel wall from the blood circulation? Is it to escape the impact of the turbulent blow damage? What is the mechanism by which it drills out? There are several theories in the literature about the mechanism by which cancer cells escape from the microvessels:
1) It is generally believed that the mechanism of cancer cells escaping from the blood vessel wall is similar to that of leukocytes in the inflammatory response, and it also goes through steps such as moving to the side, slowing down the flow rate, rolling over the wall, and swimming out.
2) Cancer cells stay in the microcirculation, and adhesion molecules play an important role in their contact with vascular endothelial cells. After cancer cells reach the target organ of metastasis, they are firmly attached to the endothelial layer of the vessel wall.
3) Capillary endothelial cells are periodically shed and renewed, and the worn or torn endothelium can form temporary fissures to expose the basement membrane, which provides a favorable basis for the adhesion of cancer cells.
4) Cancer cells interact with platelets in the process of circulatory system metastasis and entangle into clusters, which is helpful for platelet adhesion when the microvascular endothelium is damaged.
5) Another way of anchoring adhesion of cancer cells may be that some larger tumor thrombi are intercepted and captured by small blood vessels, thus implanting in metastatic organs and forming metastasis.
7. Cancer cells escape from the blood and implant in external organs
After the cancer cells penetrate the blood vessel wall, they adhere, divide and proliferate around the blood vessels and the tissue cells of the parenchymal organs, and grow locally, forming a small tumor cell clone around the blood vessel, which is called a micrometastasis. This tumor cell foci lacks stroma and is most susceptible to host factors and damage. This micrometastasis is isolated and often less than 1-3mm in diameter. It can obtain fibrous tissue and blood vessels and other stroma from normal host tissue. It is the stroma of the tumor. At this time, the cancer cells are still isolated, and most of them are in a dormant state. The number of cells is limited to less than 1,000,000. There is no new blood vessel formation at this stage, and it is limited to the implantation site and grows slowly. The micro-lesions are in a semi-quiescent state for a long time, and tumor cells can produce tumor-forming factors (FAF), such as endothelial cell growth factor, vascular permeability factor (Vascnlar endothelicum growth tactor/Vascular permeability factor, VEGF/VPF), which It can promote the formation of blood vessels and supply nutrients to tumor cells. During the growth and development of micrometastases, there must be blood vessels to provide sufficient nutrition, otherwise, the tumor may remain in an avascular phase or a “dormant” state.
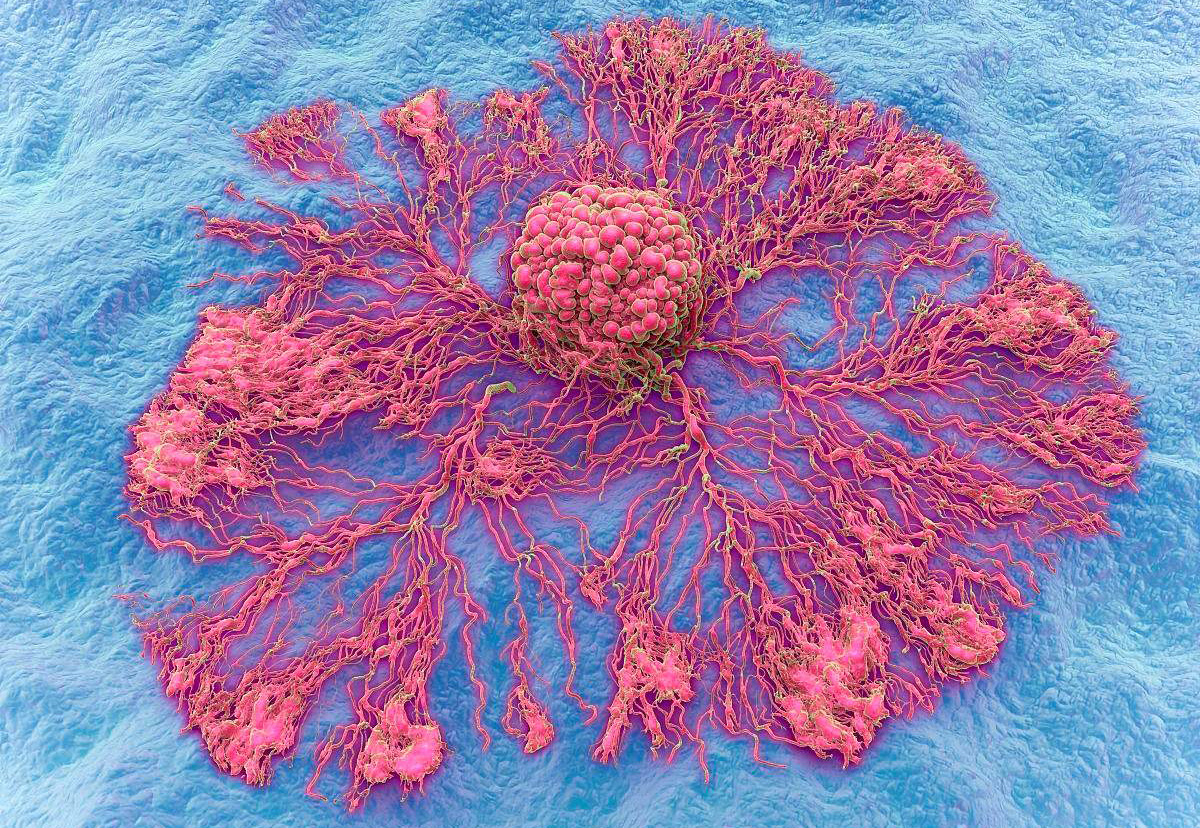
8. Angiogenesis, cancer cells proliferate to form metastases
Can cancer cells form metastases after escaping from blood vessels to reach the target organ of metastasis? A large number of research data show that angiogenesis is the premise and basis of tumor metastasis, and tumor metastasis is closely related to angiogenesis. Usually, malignant tumors without neovascularization tend to be semi-dormant, confined to the site of implantation, and grow slowly. Once tumor cells release a large amount of angiogenic growth factors, the local stable vascular bed signal is deregulated, and a large number of new blood vessels grow. Some people divide tumor angiogenesis into two phases: pre-vascularization and vascular phase. In the early stage of the formation of solid tumors, the diameter of the primary tumor is 1-3 mm, the number of cells is also limited to less than 1,000,000, and the lesions are in a static state for a long time. After the newly formed blood vessels enter the vascular phase, the tumor will grow uncontrollably. The tumor volume can reach 10,000-20,000 times the original size within 2 weeks, and a large number of tumor cells will metastasize to distant sites through blood vessels. Therefore, neovascularization is a key link in the initiation of the metastatic chain process.
Metastasis of tumors is a process that depends on the growth of blood vessels. When the tumor volume is 1-2 cubic centimeters, the maintenance of its continued growth depends on the formation of new blood vessels. These new blood vessels directly link tumor cells to the circulatory system, enabling the exchange of materials for tumor growth. In addition, neovascularization can also serve as a channel for tumor cell metastasis, transporting cancer cells to target organs through neovascularization. Therefore, angiogenesis is closely related to the formation of metastases. If the metastatic cancer cells do not form new blood vessels, they will not further enlarge to form metastases. How do tumors promote angiogenesis? Tumor tissue-induced angiogenesis is a complex, multifactorial regulated process. Many angiogenic factors have been found, such as: vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), insulin-like growth factor (1GF-1), transforming factor-a (TGF-a), Platelet-derived growth factor (PDGF), etc. These angiogenic factors degrade the vascular basement membrane and surrounding extravascular matrix through various mechanisms, promote endothelial cell division, migration and proliferation, and induce host capillaries to grow and grow into tumor tissue. Promote the formation and enlargement of metastases.