Foreword
The mucin (MUC) family is a group of highly glycosylated macromolecules that are abundantly expressed in mammalian epithelial cells. Mucins contribute to the formation of the mucus barrier and are therefore protective against infection. Interestingly, some MUC proteins are abnormally expressed in cancer cells and are involved in tumorigenesis and progression, including cell growth, proliferation, inhibition of apoptosis, chemoresistance, metabolic reprogramming, and immune evasion.
Due to their unique biological and structural features, MUC proteins are considered important biomarkers for certain cancers and very promising therapeutic targets.
Structure and classification of mucins
Mucins are divided into two categories: secretory mucins and transmembrane mucins. Secreted mucins include gel-forming and non-gel-forming mucins, including MUC2, MUC5AC, MUC5B, MUC6, MUC7, MUC8, MUC9 (OVGP1), and MUC19. Transmembrane mucins also include a transmembrane domain and an intracellular domain, including MUC1, MUC3A, MUC3B, MUC4, MUC12, MUC13, MUC14 (endothelial mucin), MUC15, MUC16, MUC17, MUC20, MUC21, and MUC22.
Transmembrane mucins are single-pass transmembrane type I membrane proteins whose N-terminal extracellular domains include tandem repeat (TR) domains, SEA (sea urchin sperm protein-enterokinase-aggregin) domains and/or EGF ( epidermal growth factor)-like domain. The TR domain contains a variable number of repetitive amino acid sequences rich in serine, threonine and proline (S/T/P). These S/T/P residues are sites for the addition of O-linked N-acetylgalactosamine (GalNAc) to initiate further N-linked glycosylation chain reactions. The TR domain, due to its highly glycosylated structure, underlies the physical and chemical characteristics of these molecules, such as lubrication or immunoprotection.
The SEA domain has a highly conserved cleavage site located near the outer side of the cell membrane. Proteolytic cleavage of transmembrane mucins separates them into an N-terminal subunit (containing the TR domain) and a C-terminal subunit (containing the transmembrane and cytoplasmic domains). The two subunits can form a non-covalent and stable complex. The EGF-like domain is homologous to some growth factor sequences and interacts with growth factor receptors such as ErbB receptors.
MUC1, the first identified mucin, also known as EMA (tumor-associated epithelial membrane antigen) or CD227, is a large glycosylated protein with an expected molecular weight between 120 and 500 kDa depending on the glycosylation status between. The variable number tandem repeat (VNTR) domain in MUC1 consists of 20–125 repeats of 20 amino acids (PAPGSTAPPAHGVTSAPDTR). MUCl also contains an SEA of 110 amino acids in length, a short transmembrane region, and an intracellular region of 74 amino acids. Its intracellular domain has several short (4-9 amino acids) protein-binding motifs that facilitate its interaction with GSK3β (glycogen synthase kinase 3β), β-catenin, GRB2 (growth factor receptor binding protein 2) , SRC and ESR1 (estrogen receptor 1) interaction. In addition, it possesses a p53 binding region, which is a relatively long 37 amino acid sequence.
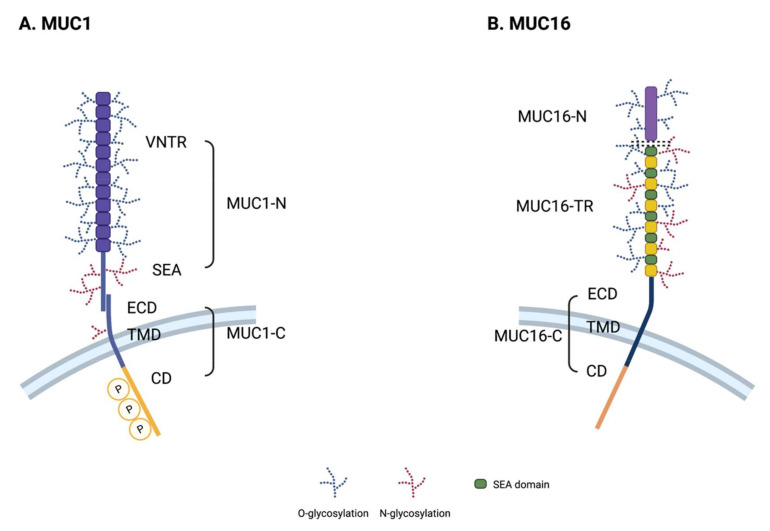
MUC16 (CA125), the largest transmembrane mucin, is a well-established serum biomarker for ovarian cancer as it is known to be overexpressed on the surface of ovarian cancer cells and to divide/shed into the blood. MUC16 contains about 14,000 amino acids and has a molecular weight between 1.5 and 5 MDa. MUC16 contains three main domains: N-terminal domain (MUC16-N), tandem repeat domain (MUC16-TR) and C-terminal domain (MUC16-C). Its N-terminal domain contains multiple serine-rich regions within a threonine-rich region about 12,000 amino acids long, which is O-glycosylated only. The TR domain consists of 12-60 repeats of 156 amino acids with interspersed SEA domains containing O-linked and N-linked glycosylation sites. The C-terminal domain of MUC16 includes an extracellular domain, a short transmembrane region, and a 32 amino acid intracellular domain.
The role of transmembrane mucins in tumorigenesis
Oncogenesis is a complex process involving various events inside and outside the cell, and many reports suggest that transmembrane mucins may play multiple roles in tumorigenesis and progression.
Promote cancer cell proliferation
One of the fundamental characteristics of cancer cells is their unlimited proliferative potential maintained by abnormal growth signaling pathways. Oncogenic mutations or overexpression of receptor tyrosine kinases (RTKs) confer constitutive activation of growth factor signaling. The cytoplasmic domain of MUC1 has multiple protein-binding motifs and phosphorylation sites, and direct interaction of RTKs with MUC1 is known to generate oncogenic signals.
ErbB is a RTK family, and MUC1 interacts with all ErbB family receptors to transmit oncogenic signals to each other. In addition, MUC1 also binds to fibroblast growth factor receptor 3 (FGFR3), another critical RTK in tumorigenesis. Upon stimulation by FGF1 ligand, FGFR3 interacts with MUC1 and phosphorylates the YEKV motif of the cytoplasmic domain of MUC1.
Anti-apoptotic effect
Another important hallmark of cancer is anti-apoptotic cell death. Cancer cells escape stress-induced apoptosis through various mechanisms, and MUC1 has a protective effect that promotes this survival. First, MUC1 attenuates DNA damage-induced genotoxic stress through the DNA replication mechanism or the action of anticancer drugs. MUC1 regulates p53-dependent gene transcription through direct association with p53 regulatory domains and p53-responsive elements. On the other hand, MUC1 directly exploits the drug efflux system by transcribing multidrug resistance (MDR) genes, which have been reported to protect lung and pancreatic cancer cells from chemotherapy.
MUC1 attenuates mitochondrial apoptotic factors, such as cytochrome c or Bcl-xL, and protects cancer cells from anticancer genotoxins such as cytarabine, gemcitabine, and cisplatin. Furthermore, MUC1 also provides a survival advantage to cancer cells by scavenging oxidative stress, MUC1 dephosphorylates and activates FOXO3a, and FOXO3a activation induces its nuclear localization and subsequent transcriptional activation of ROS scavenging genes.
MUC16 is also thought to play an anti-apoptotic role in cancer cells. Ectopic expression of the C-terminal domain of MUC16 induces resistance to cisplatin in ovarian cancer cells, an effect mediated by inhibition of p53. Although the exact molecular mechanisms of its binding protein and drug resistance phenotype are unknown, the intracellular domain of MUC16 is thought to have a signaling role comparable to that of MUC1.
Reprogramming of energy metabolism
Altered mucin expression in various cancer tissues has been suggested as a regulator of energy metabolism reprogramming. In an earlier study, MUC1 was shown to increase glucose metabolism in pancreatic cancer patients. In an orthotopic pancreatic cancer mouse model, MUC1 overexpression was also associated with increased glucose uptake and HIF-1α, GLUT1 and LDHA protein expression.
Alterations in lipid metabolism are also associated with cancer progression. One study proposed a subset of 38 genes, named MLMS (MUC1-induced lipid metabolism signal), consisting of differentially expressed genes associated with lipid metabolism in MUC1-transformed 3Y1 cells. In tamoxifen-treated breast cancer patients, MLMS overexpression was associated with poor prognosis, suggesting that altered lipid metabolism may contribute to tamoxifen resistance.
EMT and Transfer
Recently, a series of studies support the role of mucin in breast and pancreatic cancer EMT. Analysis of MUC1-overexpressing cells and knockout mouse models showed that MUC1 strongly affects EMT processes in pancreatic cancer. For example, EMT was blocked when all tyrosine residues in the intracellular domain of MUCl were replaced by phenylalanine. This MUC1 mutant cannot bind to β-catenin and thus cannot translocate to the nucleus to promote EMT gene transcription.
MUC16 is also a mediator of EMT in pancreatic cancer, and its knockdown resulted in reduced cancer cell migration in vitro and reduced metastasis in vivo. In fact, the recently described interaction between MUC16 and FAK has been suggested as a mechanism of pancreatic cancer metastasis.
Evade immune surveillance
Since mucins expressed in normal epithelial cells play an important role in mucosal immunity against bacterial infection, cancer-associated mucins are thought to regulate tumor immunity. Mucins are involved in multiple strategies to evade host immunity, including (1) blocking interactions between immune cells and cancer cells, (2) modulating immune cell signaling through co-stimulatory or co-inhibitory molecules, and (3) modulating pro-inflammatory production of cytokines. Mucins have inhibitory effects on cell-to-cell interactions due to their large glycosylated structures in their extracellular regions.
Immune cell infiltration analysis of TCGA samples showed a strong negative correlation between mucin mRNA expression and tumor cytotoxic lymphocyte infiltration. In addition, overexpressed MUC1 and MUC4 on the cancer cell surface provide a steric barrier to the association between cancer cells and cytotoxic lymphocytes, resulting in reduced cancer cell lysis. Glycosylated MUC1 on cancer cells directly binds to selectins or siglec family proteins expressed on immune cells, including macrophages, and inhibits their function. In addition, MUC1 functions as an immune checkpoint molecule by binding to intercellular adhesion molecule 1 (ICAM-1) on T cells and inhibiting its function.
MUC-targeted tumor therapy
Numerous studies have shown that mucins are promising targets for cancer therapy. Due to its role in tumor signal transduction pathways, transmembrane mucin signaling pathways may have particular potential in anti-tumor therapy research. The extracellular domains of membrane-bound mucins can also serve as good targets for antibody-mediated therapy, such as neutralizing antibodies, CAR-T, BsAbs, and ADCs. Cancer-specific expression of certain mucins also suggests the possibility of developing mucin antigen-based cancer vaccines.
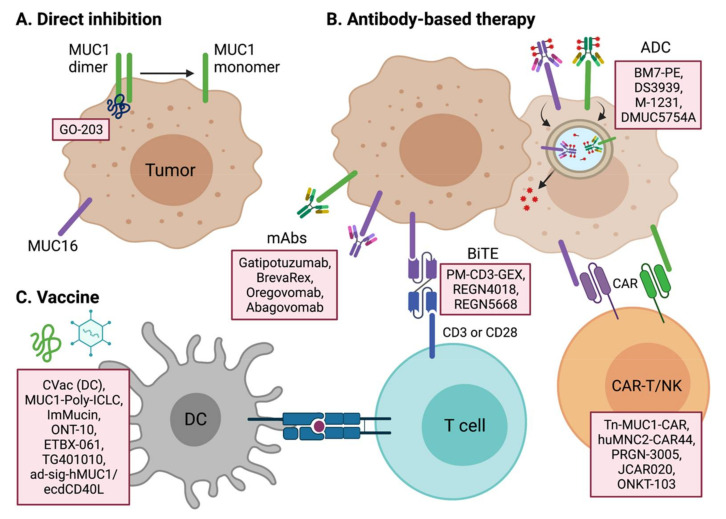
Targeted therapy of MUC1
Multiple therapeutic candidates for MUC1 are in development for multiple cancer types, including solid and hematological tumors.
GO-203 is a cell-penetrating peptide inhibitor of MUC1 dimerization that binds directly to the CQCRK region of the intracellular domain of MUC1. GO-203 has antitumor efficacy by blocking the AKT pathway in multiple tumor types. GO-203 also shows potential in combination with chemotherapy-resistant cancer cells or standard chemotherapy in refractory cancer types.
Selective RNA aptamer binding to the extracellular domain of MUC1 is another strategy to target MUC1. Antitumor microRNAs were introduced into MUC1-overexpressing cancer cells, and these miRNA-29b-loaded hybrid nanoparticles showed antitumor effects in a mouse model of lung cancer by downregulating DNMT3B. Furthermore, using a dual-payload strategy, geistein-miRNA-29b double-conjugated hybrid nanoparticles showed greater potency than single-payload nanoparticles by targeting AKT, PI3K, DNMT3B, and MCL-1 in a mouse lung cancer model.
Antibody-based therapeutics such as ADCs, BiTEs, and CAR-Ts, as well as neutralizing therapeutic antibodies, are all under active development. BM7-PE and M-1231 are the lead candidates for MUC1 ADCs currently in clinical trials. BM7-PE is an ADC conjugated with the anti-MUC1 antibody BM7 to Pseudomonas exotoxin A (PE). In a preclinical study, BM7-PE showed anti-metastatic effects and promoted long-term survival in a nude mouse model of breast cancer. BM7-PE is currently undergoing a Phase 1/2 clinical trial in metastatic colorectal cancer (NCT04550897). M-1231, an ADC of bispecific antibodies against EGFR and MUC1, is currently undergoing Phase 1 clinical trials in various metastatic solid tumors.
Pab-001 is the first class of therapeutic antibody against OT-MUC1 (tumor tethered MUC1). Pab-001-MMAE ADC has shown promising results against TNBC and other cancers in various preclinical settings. DS-3939 is a PankoMab GEX (gatipotuzumab) ADC targeting the tumor-specific mucin carbohydrate-protein epitope (TA-MUC1), and a bispecific antibody using PankoMab is being developed concurrently. PM-CD3-GEX is a BiTE that recruits antitumor CD3+ T cells to MUC1-expressing cancer cells. PM-IL15-GEX combines IL-15 with PankoMab GEX to simultaneously stimulate anti-tumor NK or T cells. PM-PDL-GEX is a trifunctional antibody against MUC1, PD-L1 and FcγR.
Several CAR-T therapies targeting the MUC1 antigen are also currently being developed. Tn-MUC1-CAR, developed by Tmunity Therapeutics, is the leading MUC1-CAR-T cell therapy and is currently undergoing Phase 1 clinical trials (NCT04025216). Since Tn (GalNAcα1-O-Ser/Thr) is the most common abnormal carbohydrate in cancer tissues, the Tn carbohydrate of MUC1 (Tn-MUC1) is a promising target for CAR therapy. Tn-MUC1 CAR-T has shown targeted antitumor efficacy against T-cell lymphomas and pancreatic tumors.
The MUC-1 pCAR developed by Leucid Bio is a parallel CAR platform that introduces two chimeric antigen receptors with different antigen binding domains and costimulatory domains, respectively (WO2020183158). This combination of dual receptors is expected to make T cells more specific for MUC1-positive tumors and more potent than standard CAR-T.
huMNC2-CAR44 T cells developed by Minerva Biotechnologies Corp against the split form of MUC1 on solid cancer cells, huMNC2-CAR44 is in Phase 1 clinical trials (NCT04020575) in breast, ovarian, pancreatic and lung cancers, all of which are MUC1 High-expressing tumor types.
ONKT-103 is a MUC1-targeted CAR-NK cell therapy developed by ONK Therapeutics. ONKT-103 maximizes its anti-tumor activity by introducing the DR5-TRAIL variant death receptor signaling pathway. ONKT-103 is currently in the preclinical stage.
MUC16 and other mucin-targeted therapies
Like MUC1, other membrane-bound mucins are also considered potential targets for anticancer therapy.
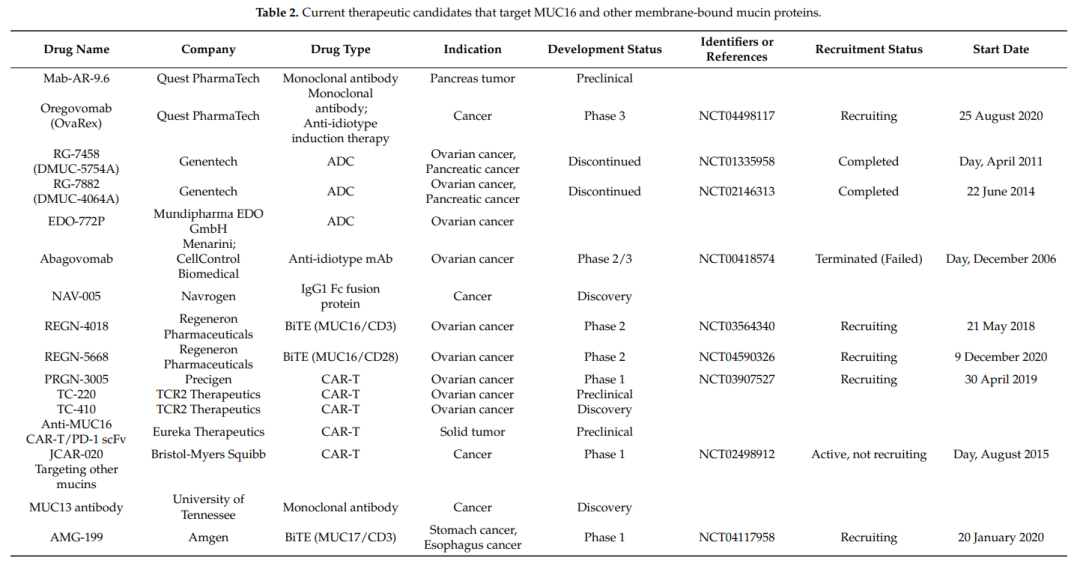
Oregovomab (OvaRex) is the first monoclonal antibody drug studied in clinical trials. Oregovomab binds to the glycosylated region of MUC16 with high affinity and induces an indirect immune response via an anti-idiotypic antibody cascade. A phase 2 clinical trial of Oregovomab in combination with carboplatin and paclitaxel (CP) in 97 patients with stage III/IV ovarian cancer showed very promising results. Progression-free survival (PFS) was 41.8 months in the CP plus oregovomab group compared with 12.2 months in the CP group (p=0.0027). The combined administration of CP and oregovomab resulted in the increase of MUC16-specific IFN-γ+CD8+ T lymphocytes in peripheral blood. However, despite encouraging results from combination studies with standard chemotherapy, oregovomab monotherapy has not shown clinical benefit in phase 2 and 3 clinical trials. Another Phase 3 clinical trial of oregovomab (NCT04498117) is ongoing.
Abagovomab is an anti-idiotypic antibody raised against the anti-MUC16 antibody OC125. Abagovomab induces a specific immune response, which in turn activates a cytotoxic response against MUC16-expressing cancer cells. As an ovarian cancer vaccine for maintenance therapy, abagovomab improved immune responses and overall survival (OS) in a phase 1b/2 trial (23.5 months vs. 4.9 months; p<0 .001). However, a multicenter phase 3 clinical study (NCT00418574) involving 888 patients failed to demonstrate these clinical benefits.
DMUC5754A (RG-7458, sofituzumab-vedotin) is an ADC containing a humanized anti-MUC16 antibody conjugated to MMAE. A phase 1 study in patients with platinum-resistant ovarian cancer (OC) and unresectable pancreatic cancer (PC) showed that despite the safety profile of DMUC5754A, the response rate in OC patients was only 17% (5/29; 1 CR; 4 PRs), no CR or PR was observed in PC patients.
Regeneron is currently developing a BiTE of MUC16, and in mouse and monkey models, REGN4018 has shown MUC16-dependent antitumor efficacy and good tolerability. REGN4018 is currently undergoing Phase 2 clinical trials, alone or in combination with PD-1 antibody cemiplimab or REGN5668 (MUC16/CD28 BiTE) in patients with recurrent ovarian cancer (NCT03564340, NCT04590326).
JCAR-020, developed by Juno/Celgene/Bristol-MyersSquibb, is a MUC16CAR-T cell therapy containing an IL-12 receptor agonist. JCAR-020 is currently undergoing a Phase I clinical trial (NCT02498912).
Besides MUC1 and MUC16, other transmembrane mucins have also been evaluated as potential cancer targets. Amgen is developing a BiTE targeting CD3 and MUC17 for the treatment of gastric and esophageal cancers in Phase 1 trials (WO2019133961A1, NCT04117958).
Tumor vaccine
In addition to mucin-targeted passive immunotherapy, mucin antigen vaccines have also been actively attempted to treat various solid tumors.
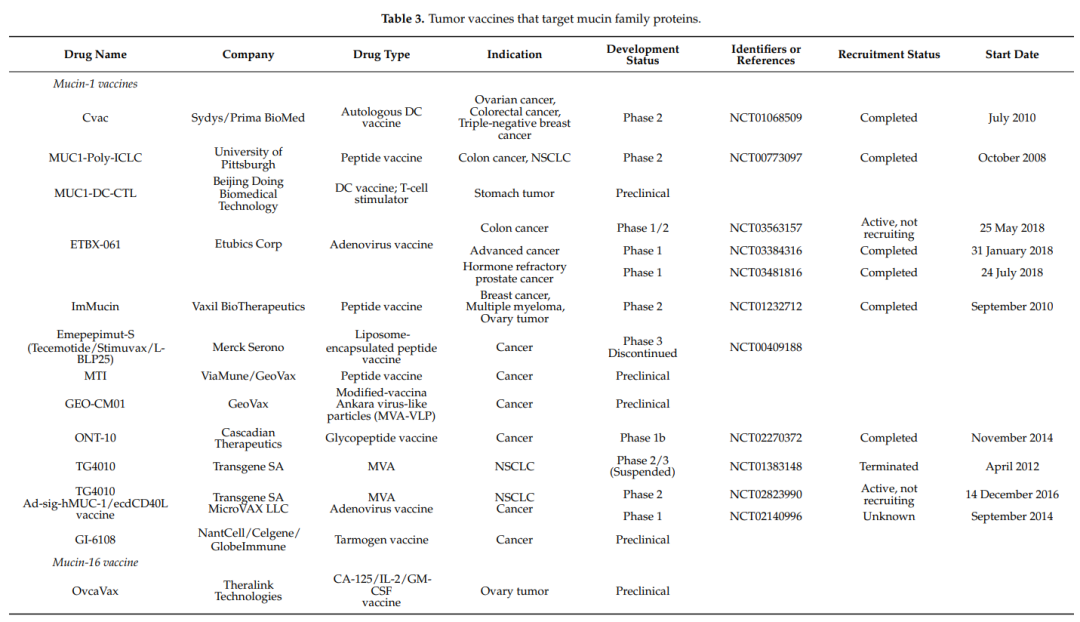
CVacs are autologous monocyte-derived DCs induced with mannosylated MUCl protein. Two phase 2 clinical studies of these cells have been conducted: one for advanced OC patients whose disease has progressed after standard chemotherapy, and the other for maintenance therapy after clinical remission in OC patients. The CVac DC vaccine was found to be sufficiently safe with minimal side effects, but failed to increase PFS compared to standard chemotherapy alone.
ImMucin is a 21-peptide vaccine containing the signal peptide domain of the MUC1 protein, which binds to various MHC class I and II alleles. A phase 1/2 study of the combination of ImMucin and GM-CSF in multiple myeloma showed that the vaccine was safe and tolerable, successfully induced vaccine-mediated cellular and humoral immune responses, and clinical disease was controlled in 11/15 patients.
ONT-10 is a liposomal therapeutic vaccine consisting of two repeats of a 20-mer synthetic glycopeptide from MUC1 with pentaerythritol lipid a (TLR4 agonist). Preclinical studies of ONT-10 have demonstrated induction of cellular and humoral immune responses to MUC1 and antitumor effects in isogenic B16-MUC1 and MC38-MUC1 models. A phase 1 study in 28 patients with advanced solid cancer demonstrated that ONT-10 was safe and well tolerated, but no CR and PR were observed. Recently, a phase 1b study (NCT02270372) of ONT-10 in combination with varlilumab (an anti-CD27 agonist antibody) was conducted in patients with advanced ovarian and breast cancer.
Emepepimut-S (L-BLP25) is another developed peptide vaccine against MUC1. However, in a phase 3 study in patients with non-small cell lung cancer, no significant difference in OS was observed.
ETBX-061 is a therapeutic adenovirus vaccine targeting the MUC1 protein. Considering the heterogeneity of solid tumors, ETBX-061 has been studied in combination with other vaccines or therapeutics in clinical trials. A triple (CEA/MUC1/Brachyury) vaccine combination regimen was investigated in a phase 1 clinical trial in patients with advanced cancer, which demonstrated antigen-specific T cell generation and disease control (60% SD and 40% PD).
TG4010 is a modified Ankara vaccinia (MVA) expressing MUC1 and IL-2. In a phase 2b/3 trial in advanced non-small cell lung cancer, TG4010 plus chemotherapy resulted in a significant improvement in PFS compared with placebo plus chemotherapy, but minimal survival benefit (5.9 months vs. 5.1 months).
MicroVAC LLC is developing an ad-sig-hMUC1/ecdCD40L vaccine in which MUC1 is fused to the extracellular domain of the CD40 ligand (CD40L) to promote DC activation and promote T and B cell expansion. A small cohort Phase 1 study of the vaccine showed that it was safe and had encouraging antitumor activity.
Summary
Transmembrane mucins have important roles in maintaining mucosal structure and physiological homeostasis. Mucins are highly glycosylated proteins that are overexpressed in different types of cancer. Efforts have been made to find new therapeutic strategies to exploit certain transmembrane mucins as therapeutic targets. Numerous mucin-targeting therapeutics are in various stages of clinical trials for several cancers, including antibody-based therapies, small molecule inhibitors, vaccines, and cell therapies. To develop more effective mucin-based therapeutic strategies, a better understanding of mucin glycoprotein shedding mechanisms, aberrant glycosylation, possible splice variants, oncogenic signaling cascades, and interacting binding proteins is required.