On June 24, BMS announced that the FDA approved a new indication of the CD19 CAR-T therapy Breyanzi (lisocabtagene maraleucel) for the second-line treatment of adult patients with large B-cell lymphoma (LBCL), including diffuse large B-cell lymphoma (DLBCL), High-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, follicular lymphoma grade 3B. These patients were refractory to first-line chemoimmunotherapy or relapsed within 12 months and were not candidates for hematopoietic stem cell transplantation (HSCT) due to age and comorbidities.
LBCL is an aggressive blood cancer that is difficult to treat. About 40% of patients develop relapsed or refractory disease after their first treatment. Historically, the only potential curative therapy consisted of initial aggressive immunochemotherapy followed by high-dose chemotherapy or hematopoietic stem cell transplantation. However, about half of the patients were unable to receive stem cell transplantation due to age and comorbidities. Treatment options for patients who cannot receive stem cell transplantation are very limited. Without treatment, patients with relapsed/refractory LBCL have a life expectancy of only 3-4 months.
Breyanzi is an autologous CAR-T therapy that was approved for the first time in the United States and the European Union in early 2021 for LBCL patients who have received two or more systemic treatments. The global sales in 2021 will be US$87 million. After the approval of the new indication, Breyanzi has become the most widely covered CAR-T cell therapy for patients with refractory or relapsed LBCL, and it is also a landmark breakthrough for large B-cell lymphoma in the past 30 years.
For patients with LBCL who relapsed or did not respond to first-line therapy (primary refractory) within 12 months of first-line therapy, Breyanzi compared with standard therapy in event-free survival (EFS), complete response rate (CR) and progression-free survival Clinically significant and statistically significant improvements were seen in PFS.
In the pivotal Phase 3 clinical trial, a single Breyanzi infusion significantly prolonged event-free survival (EFS) in patients compared to standard care, with EFS of 10.1 months in the Breyanzi arm and 2.3 months in the control arm. Complete remission was achieved in 66% of patients in the Breyanzi group, compared with 39% in the control group. Trial results also showed that Breyanzi extended the progression-free survival of patients by more than 2 times.
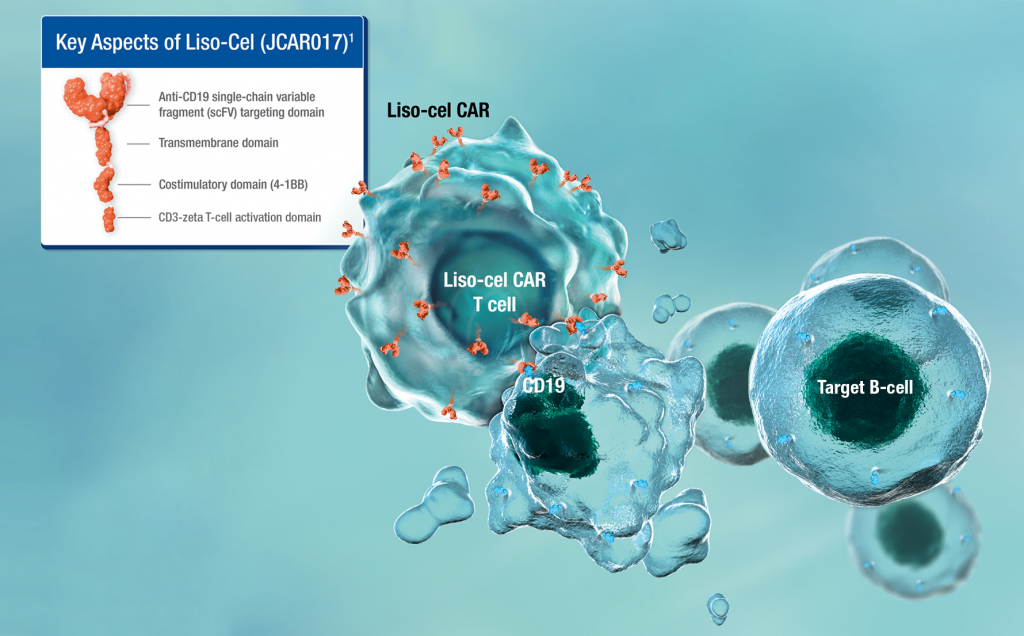
Breyanzi has a better safety and tolerability profile with less CRS and neurotoxicity than other Yescarta and Kymriah, which also achieved higher response rates in large B-cell lymphomas.
In the TRANSFORM study, all-grade CRS occurred in 45% of the Breyanzi arm and grade 3 CRS was 1.3%; all-grade neurotoxic events occurred in 27% and grade 3 or higher in 7%. The safety advantage of Breyanzi is that it introduces 4-1BB as a costimulatory signal. Compared with CAR-T cells that introduce CD28 as a costimulatory signal, it has the advantages of being more durable, less prone to exhaustion, slower and more controllable in expansion. .
At present, there are 8 CAR-T cell therapies on the market in the world, and BMS and Gilead have two exclusives each. The approval of this indication further consolidates BMS’ leading position in the field of cell therapy.