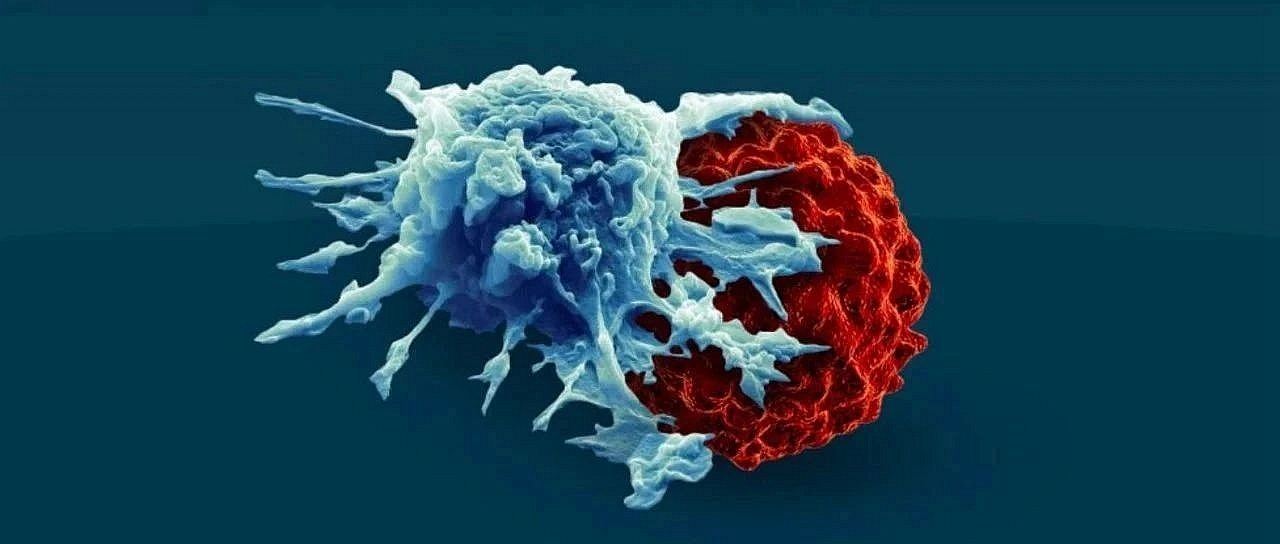
As a new type of immunotherapy, CAR-T cell therapy boosts a patient’s killer T cells to attack and eliminate cancer. It can be as high as 90% effective against certain blood cancers, and even enables long-term remission and cure in some patients. But an important limitation is the therapy’s harmful side effects, with dangerous complications occurring in about 50 percent of patients.
In a new study, researchers from the Weizmann Institute of Science in Israel, the Walter-Elisa Hall Institute for Medical Research in Australia, the University of Melbourne and La Trobe University have developed a potential reduction in CAR-T The discovery of the toxic side effects of cell therapy may overcome the greatest limitation of this pioneering therapy. To do so, they used this one approach to identify a “golden” window that strikes a balance between safety and efficacy. Their approach fine-tuned the CAR-T cells used in this immunotherapy so that they were active enough to eliminate the cancer, but not strong enough to cause toxic side effects. The findings were recently published in the journal eLife under the title “De novo-designed transmembrane domains tune engineered receptor functions”. The corresponding authors of the paper are Associate Professor Matthew Call, Associate Professor Melissa Call, and Sarel J Fleishman of the Weizmann Institute of Science.
Critical redesign
CAR-T cell therapy involves harvesting T cells from cancer patients, enhancing the capabilities of those cells by reengineering them in the laboratory, and then putting these enhanced T cells back into the patient. These redesigned T cells produce proteins on their surfaces called chimeric antigen receptors (CARs), which act as artificial sensor proteins that allow T cells to more efficiently recognize and bind to specific proteins on the surface of cancer cells .
It is this artificial sensor protein that gives T cells a greater ability to attack and eliminate threats, such as cancer cells, Matthew said. “While putting these ultra-potent T cells into patients with higher tumor burdens can rapidly destroy cancer cells, it also creates a potentially harmful cytokine storm because of the ongoing toxic response.”
There is currently no way to reliably predict how well CAR-T cell therapy will work in patients. Previous studies have attempted to fine-tune T cells by targeting end portions of this artificial sensor protein that either bind to cancer cells or instruct T cells to kill. In contrast, in the new study, the authors are the first to completely redesign the middle part of this artificial sensor protein, or CAR: the transmembrane domain (TMD). Specifically, they developed a de novo design strategy to generate programmed membrane proteins (proMPs) — single-channel alpha-helix TMDs that self-assemble through computationally defined and crystallographically validated interfaces. . They used these proMPs to program specific oligomeric interactions into CARs and expressed them in mouse primary T cells, and found that both the in vitro cytokine release and in vivo antitumor activity of CAR-T cells were correlated with low CAR TMD-encoded The aggregation state (from monomer to tetramer) is linear. Compared to the commonly used CD28 TMD, all T cells stimulated with programmed CARs have greatly reduced cytokine release.
The authors leveraged the computational expertise of the Weizmann Institute of Science to stitch together fragments of innate immune sensor proteins with custom-designed synthetic elements to generate new circuits that can be used to tune and assess changes in potency. Matthew said, “Focusing on the intermediate junction segment of this artificial sensor protein allows us to generate different versions of CARs that we know are stronger or weaker, which allows us to tailor them to a patient’s potency requirements. Predictable Tuning the activity of this T cell significantly broadens our scope, unlike previous studies, because we target certain targets that are present in every immunotherapy setting. For the first time, we can build Rules for treating cancer with CAR-T cell immunotherapy.”
Intensive treatment
Associate Professor Melissa Call said the ability to fine-tune T cells would greatly reduce the number of patients experiencing serious side effects from the treatment, which could include fever, high blood pressure and difficulty breathing. “CAR-T cell therapy has been shown to be effective in eradicating very advanced leukemias and lymphomas, while keeping the cancer under control for years — even after patients stop taking their cancer drugs. This therapy has incredible potential for cancer patients. therapeutic potential, but is currently used as a last resort due to these potentially serious side effects. Our approach may fundamentally rethink the way CAR-T cell therapy is delivered, reducing the risk of patients being exposed to harmful side effects. This will enable widespread of cancer patients receive CAR-T cells earlier in the treatment process.”
There are more than 600 clinical trials of CAR-T cell immunotherapy, and this type of immunotherapy has been used to treat a variety of blood cancers. The authors hope their new approach might be used to triage patients treated with immunotherapy based on the level of efficacy desired in the early stages of treatment, and bring the field closer to finding a “golden” treatment window for many different cancers.