When it comes to cancer, everyone is very worried. The incidence and mortality of cancer worldwide are rising. According to statistics [1], the global cancer incidence rate in 2020 will be 247.5/100,000, and the mortality rate will be 1.278/100,000. At present, the treatment methods for cancer prevention and treatment include surgery, radiotherapy and chemotherapy, targeted therapy and immunotherapy. However, a considerable proportion of patients cannot be effectively treated. Cell-based therapies have revolutionized cancer treatment in recent years.
A recent review published in the journal “Biomedicines”[2] details the research progress of chimeric antigen receptor cell therapy (including CAR-T, CAR-NK) in the field of cancer treatment and its application in different tumors; The unique ability of mesenchymal stem cells (MSCs) as tumor therapeutic vehicles and the possibility to enhance the activity of CAR immune cells. Help everyone understand the development of different types of cells in the field of cancer treatment.
Cell therapy, also known as adoptive cell therapy, is a major research hotspot in the field of cancer treatment in recent years. Among them, the most concerned are CAR-T and CAR-NK cells. This therapy first collects T/NK cells from patients/healthy donors and transforms them in vitro so that they can specifically target the surface of tumor cells. The receptor for the antigen is equivalent to putting a GPS on the cells, and then re-injecting these modified cells into the patient, so that it can more accurately target tumor cells.
Although great progress has been made in CAR-T and CAR-NK cell research, especially in the treatment of hematological malignancies, challenges in solid tumors due to toxicity, off-target, and limited stability remain to be resolved.
To solve the above problems, researchers introduced mesenchymal stem cells (MSCs) as biological carriers, which can enhance the targeting and activity of CAR-cells by virtue of their homing effect to tumor tissue.
CAR-cell clinical research progress
Everyone must be familiar with CAR-T cell therapy. About 1,000 related clinical trials are underway, and many products have been approved for marketing. At present, the focus of CAR-T cell therapy is to address related side effects, including cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and off-target toxicity.
In recent years, the emergence of CAR-NK cells is considered to be expected to solve the above problems. At present, 29 CAR-NK cell therapy studies have been carried out around the world.
Compared with CAR-T cells, CAR-NK cells are not inhibited by Tregs cells, and when CAR-NK cells are activated, they can perform cytotoxic activities without prior stimulation with tumor antigens; another advantage is that It does not stimulate graft-versus-host disease (GVHD) and is expected to achieve allogeneic applications; in addition, CAR-NK cells do not cause side effects such as CRS.
For CAR-T cells, a phase 1 clinical trial[3] conducted CAR-T cell therapy in 23 cancer patients (including 14 with hepatocellular carcinoma, 7 with pancreatic cancer, and 2 with colorectal cancer). The results showed that after receiving treatment, 3 patients had partial remission and 14 patients had stable disease. Twenty-one patients had no detectable new lesions, and biopsies showed the elimination of cancer cells expressing specific antigens.
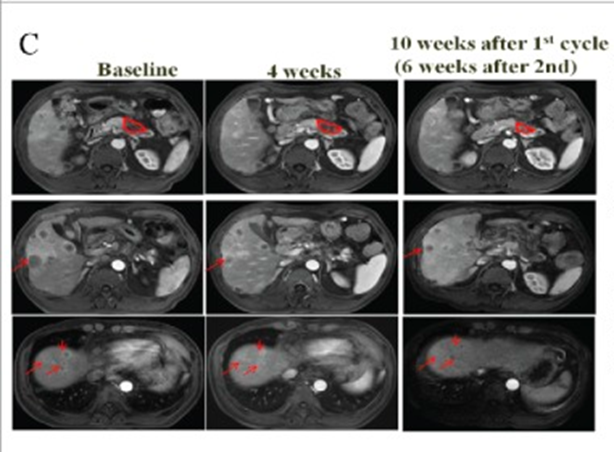
Figure 1 Imaging changes of the patient before and after treatment (Source: [3])
For CAR-NK cells, a study[4] treated 3 patients with metastatic colorectal cancer with CAR-NK cells, two patients received low-dose intraperitoneal infusion of CAR-NK cells, and the ascites decreased, The number of tumor cells in the ascites specimen was reduced, and a third patient with liver metastases received ultrasound-guided percutaneous injections with rapid tumor regression.
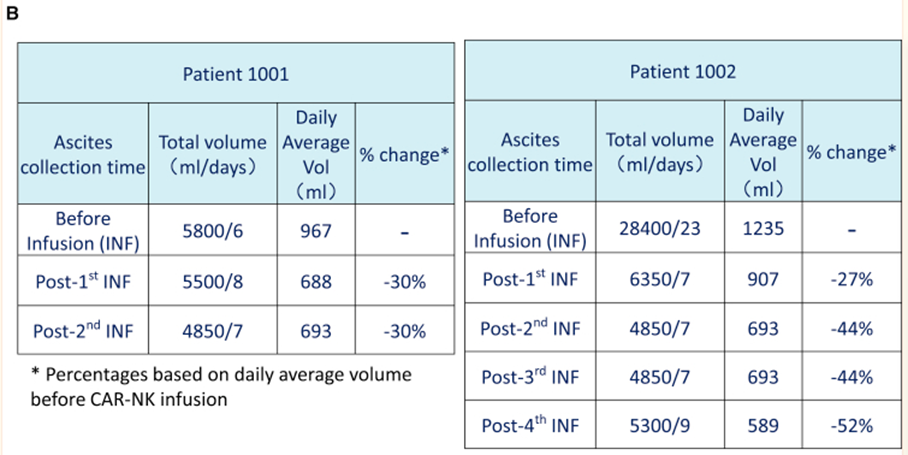
Figure 2 Changes in the volume of ascites in patients before and after treatment (Source: [4])
MSCs: a good helper for solving CAR-cell deficiency
Although CAR-T and CAR-NK cells have achieved remarkable results, their shortcomings also exist. The most unresolved ones are the exhaustion of cells and the poor efficacy in the treatment of solid tumors, which leads to the need to explore other new methods to improve their efficacy.
In recent years, mesenchymal stem cells (MSCs) have been gradually used in synergy with CAR-cells to solve the problem of insufficient CAR-cells.
MSCs can secrete a variety of chemokines and cytokines, which can initiate an acute immune response, thereby maintaining the activity of CAR-T cells; in addition, MSCs can be used as carriers to deliver biologically active proteins (supportive cytokines) and transgenic immune regulation to tumor tissues agents, etc., which causes the tumor bed to shift from an initial suppressive state to an immunostimulatory environment that supports CAR-T cell activity and enhances its anti-tumor invasiveness.
At present, relevant clinical studies on MSCs improving the efficacy of CAR-cells have not been carried out, but a considerable number of animal experiments have shown their great potential. For example, in colorectal cancer [5], MSCs are designed to release the cytokines IL-7 and IL-12 in the tumor, and IL-7 can promote the homeostatic expansion of T cells and maintain the memory cell function of T cells. IL-12 can induce protective Th1 responses, prevent Th2 polarization of T cells, and activate innate immune responses to eliminate cancer cells that CAR-T cells cannot eliminate.
Summary and Outlook
With the continuous research of CAR-cells, it is now possible to maximize the potential of CAR-cells by identifying the best tumor-associated antigens. However, it has both advantages and limitations, such as low cell targeting, poor persistence, and an immunosuppressive environment. The above problems are expected to be overcome by the application of MSCs, which have various potentials ranging from providing a variety of antitumor drugs (including oncolytic viruses and tumor-specific prodrugs) to introducing immunomodulatory proteins (such as chemokines and interleukins). Not only enhances the efficacy of CAR-related immunotherapy, but also provides a promising future for solid tumor therapy. In the future, with the combined application of CAR-cells and MSCs, it is expected to provide another kind of treatment hope for patients with solid tumors!
References:
[1] International Agency for Research on Cancer. Global cancer observatory: cancer today [EB/OL]. [2022-06-22]. https://gco.iarc.fr/today/online-analysis-table
[2]Chan LY, Dass SA, Tye GJ, et al. CAR-T Cells/-NK Cells in Cancer Immunotherapy and the Potential of MSC to Enhance Its Efficacy: A Review. Biomedicines. 2022;10(4):804. Published 2022 Mar 30. doi:10.3390/biomedicines10040804
[3] Wang Y, Chen M, Wu Z, et al. CD133-directed CAR T cells for advanced metastasis malignancies: A phase I trial. Oncoimmunology. 2018;7(7):e1440169. Published 2018 May 7. doi:10.1080 /2162402X.2018.1440169
[4] Xiao L, Cen D, Gan H, et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther. 2019;27(6):1114-1125. doi:10.1016/j .ymthe.2019.03.011
[5] Hombach AA, Geumann U, Günther C, et al. IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells. Cells. 2020;9(4):873. Published 2020 Apr 3. doi: 10.3390/cells9040873









