Colorectal cancer (CRC) is one of the most common malignant tumors, and its prevalence ranks third among malignant tumors. Despite the success of new targeted drugs for other cancers, biomarkers and targeted drugs for colorectal cancer are relatively scarce.
In April of 2022, the GCC19CART from Shanghai-based Steansai Company (ICT) was granted Fast Track status by the US FDA. GCC19CART is a CAR-T cell therapy based on ICT’s unique technology platform, CoupledCAR®, which targets guanylate cyclase C (GUCY2C) in the treatment of refractory metastatic colorectal cancer (R/R mCRC) [1].
A previous proof-of-concept (PoC) human trial of GCC19CART in China has yielded positive results, and the investigators have enrolled 35 patients with advanced colorectal cancer in an early-stage clinical trial (IRB) initiated in China, in a dose-escalating trial. In 8 patients who received GCC19 CART at a dose of 2 × 10 cells/kg, an objective response rate of 50% was observed. Based on previous positive results, the FDA in August approved a Phase I multicenter study evaluating the safety and tolerability of GCC19CART in subjects with relapsed or refractory metastatic colorectal cancer.
It can be seen that GCC19CART, the targeted drug of GUCY2C, has great potential in the immunotherapy of R/R mCRC patients. As an emerging target of CAR-T therapy in recent years, the targeted drug of GUCY2C has great potential application value.
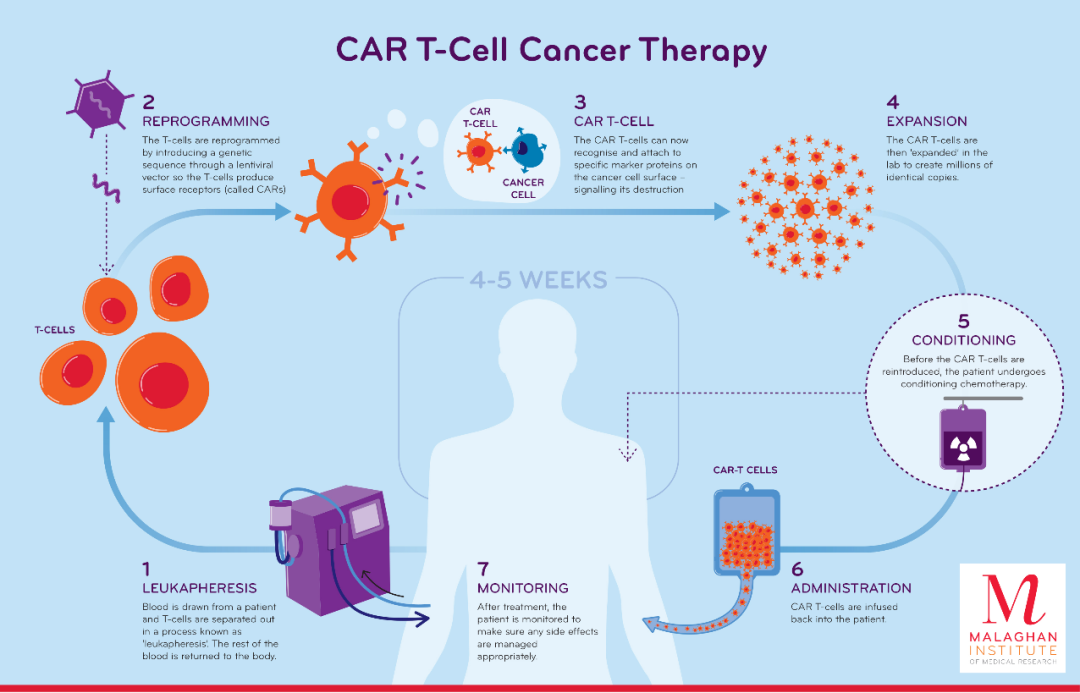
Figure 1 CAR-T therapy (Chimeric Antigen Receptor T-Cell Immunotherapy)
Structure and function of GUCY2C
Guanylate cyclase C (GUCY2C, commonly known as GC-C, or STa receptor, STaR) is an enzyme encoded by the GUCY2C gene in humans and belongs to the receptor guanylate cyclase family.
GUCY2C is selectively expressed by enterocytes and is present at the membrane apex of enterocytes from the duodenum to the distal rectum. Its protein structure consists of five main parts:
①Extracellular ligand binding domain;
②The hydrophobic transmembrane domain transmits extracellular signals into the cell;
③The cytoplasmic domain, which transmits the signal to the catalytic region;
④ catalytic zone;
⑤ Carboxyl terminal region [2, 3].
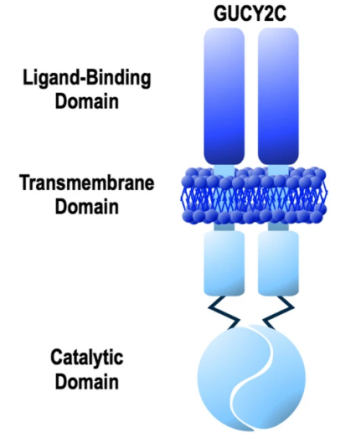
Figure 2 Structure of GUCY2C
In normal physiology, GUCY2C acts as a tumor suppressor, catalyzes the production of the intracellular second messenger cyclic guanosine monophosphate (cGMP) upon binding of its paracrine hormones guanosine or uroguanosine, restricts epithelial cell transformation, and functions in vivo to regulate in vivo Balance, maintain intestinal barrier function and exert anti-inflammatory effects [3,,4].
GUCY2C-cGMP Signal Axis
Current studies show that activation of the receptor GUCY2C by its ligand increases intracellular cGMP levels. The cGMP effector PKGII inhibits the uptake of sodium ions by the NHE3 transporter and promotes the secretion of anions by the CFTR transporter, resulting in an electrolyte and fluid gradient into the intestinal lumen. Cystic fibrosis transmembrane conductance regulator (CFTR) is further enhanced by inhibiting dual-specificity phosphodiesterase III (PDE3), cGMP accumulation, and cross-activating cAMP/PKA signaling [5]. Therefore, the GUCY2C-cGMP signaling axis can maintain normal intracellular and extracellular ion concentrations and fluid and electrolyte balance, and maintain normal intestinal function.
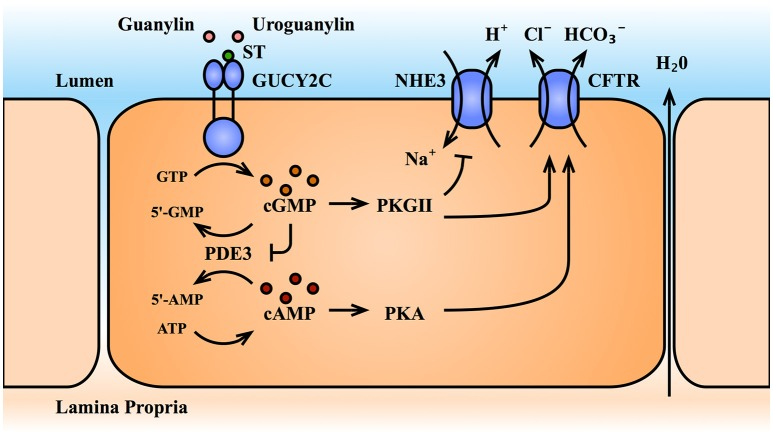
Figure 3 GUCY2C-cGMP signaling axis
GUCY2C targeting strategy
GUCY2C regulates homeostatic circuits that are often dysregulated during tumorigenesis. Loss of GUCY2C ligands, ie silencing of receptor signaling, is a very common step early in tumorigenesis, leading to genomic instability, metabolic reprogramming, and uncontrolled proliferation [6]. Endogenous GUCY2C-activating ligands are lost in early stages of transformation as well as in chronic inflammatory epithelial cells, suggesting a mechanistic basis for this putative colorectal cancer risk factor.
GUCY2C is abnormally highly expressed in metastatic colorectal cancer cells and stably expressed in primary colorectal cancer cells. GUCY2C is strongly expressed in peripheral blood of colorectal cancer patients, suggesting that GUCY2C can be used as an early detection indicator for postoperative recurrence and metastasis of colorectal cancer patients [7]. Therefore, GUCY2C may be a specific marker molecule for metastatic colorectal cancer. The expression of GUCY2C in metastatic cancer cells provides a biomarker for immunotherapy, including vaccines, CAR-T cells, and immunotoxins.
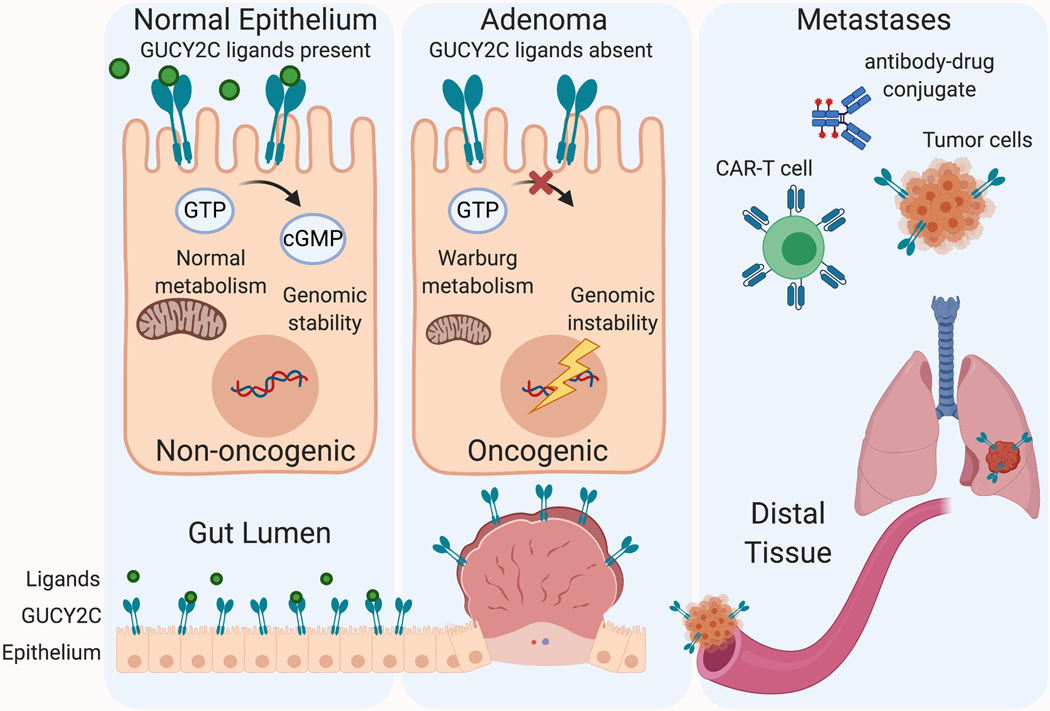
Figure 4 GUCY2C targeting strategy
Therapeutic significance of GUCY2C in colorectal cancer
The efficacy of targeting the GUCY2C-cGMP axis for tumor prevention was first demonstrated by Shailubhai et al. [8]. Currently, GUCY2C agonists have been shown to combat cell proliferation, genomic instability, barrier dysfunction, inflammation, dysbiosis, connective tissue hyperplasia, and other factors that contribute to tumorigenesis in addition to the above (Table 1).
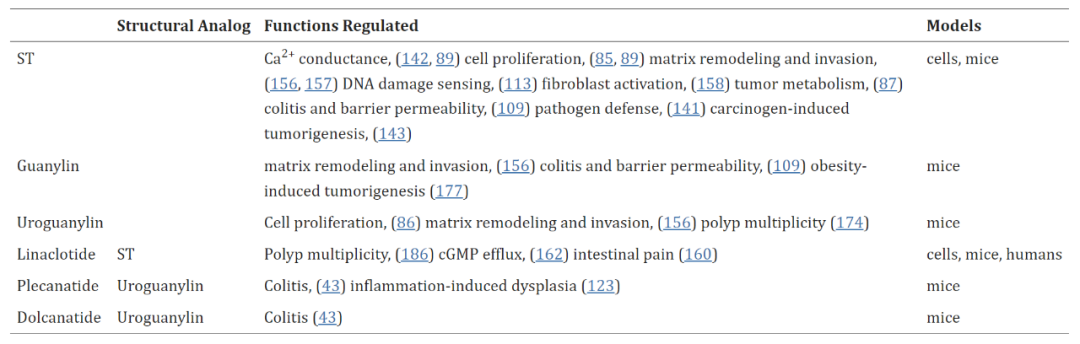
Table 1 GUCY2C agonists inhibit tumorigenic cells
Clinical research and prospect of GUCY2C targeting drug GCC19CART
The GCC19CART is the lead product candidate for ICT’s CoupledCAR ® technology. It has been tested in 35 patients in an IRB-approved Chinese trial. Data from the trial have been presented at the 2022 ASCO, ASGCT and AACR meetings.
At the annual meeting of the American Society of Clinical Oncology (ASCO) held in June 2022, ICT companies will report the latest data on GCC19CART in the form of a poster. According to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1), the overall objective response rate (ORR) for both dose levels was 28.6% (6/21), with an ORR of 15.4% in the tier 1 dose group and 50% in the tier 2 dose group. The remaining 4 subjects were also observed to respond well, with a disease control rate (DCR) of 100% within 3 months. GCC19 CART demonstrated meaningful dose-dependent clinical activity and acceptable safety in relapsed or refractory metastatic colorectal cancer.
ICT had announced in August that the first patient had been enrolled in its Phase I trial of GCC19CART in R/R mCRC. The study (NCT05319314) is an open-label, single-arm, multicenter, phase I, dose-escalation clinical trial evaluating the safety, tolerability, efficacy, and drug efficacy of GCC19CART in patients with relapsed or refractory metastatic colorectal cancer. Kinetics (PK) and Pharmacodynamics (PD).
“This is an important and exciting milestone for ICT,” said ICT CEO Dr. Larry Lei Xiao. “There is a huge unmet medical need for R/R mCRC patients. We believe GCC19CART has the potential to provide significant clinical benefit to these patients.”
Research progress of other GUCY2C drugs
GUCY2C is also being studied in several immunotherapies, such as vaccines, bispecific antibodies, and immunotoxins. Pfizer’s preclinical studies have shown that GUCY2C-CD3 bispecific antibody PF-0706119 has good antitumor efficacy[9]. At present, preclinical studies of PF-07062119 are still in progress, and its efficacy needs to be further studied in clinical trials verify. In addition, the Sidney Kimmel Cancer Center at Thomas Jefferson University has developed a colorectal cancer-specific vaccine, Ad5-GUCY2C-PADRE, which links GUCY2C to PADRE to enhance the immune response. Preclinical studies have shown that the vaccine can activate CD8+ T cells to generate an immune response and prevent colorectal cancer metastasis [10].
summary
As an important member of the intestinal signaling system, GUCY2C has a certain theoretical basis for the GUCY2C-cGMP axis as a colorectal cancer prevention and treatment target. Recent GUCY2C targeting studies have also highlighted its important role in colorectal cancer. Therefore, the application of GUCY2C CAR-T therapy or vaccine, such as GCC19CART, to colorectal cancer is a major breakthrough. Advances in the development of new immunotherapies will surely bring new insights into the treatment of gastrointestinal malignancies, especially colorectal cancer.
References:
0.Integle
1. Chen, Naifei, et al. “Dose Escalation Study of GCC19CART CoupledCAR® Technology for Patients with Relapsed or Refractory Colorectal Cancer.” Blood 138.Supplement 1 (2021): 4841-4841.
2. Flickinger Jr, John C., et al. “Guanylyl cyclase C as a biomarker for immunotherapies for the treatment of gastrointestinal malignancies.” Biomarkers in Medicine 15.3 (2021): 201-217.
3. Basu, Nirmalya et al. “Intestinal cell proliferation and senescence are regulated by receptor guanylyl cyclase C and p21.” The Journal of biological chemistry vol. 289,1 (2014): 581-93.
4. Hasegawa M, Hidaka Y, Matsumoto Y, Sanni T, Shimonishi Y. Determination of the binding site on the extracellular domain of guanylyl cyclase C to heat-stable enterotoxin. J Biol Chem. 1999 Oct 29;274(44):31713 -8.
5. Rappaport, Jeffrey A., and Scott A. Waldman. “The guanylate cyclase C—cGMP signaling axis opposes intestinal epithelial injury and neoplasia.” Frontiers in oncology 8 (2018): 299.
6. Rappaport, Jeffrey A, and Scott A Waldman. “An update on guanylyl cyclase C in the diagnosis, chemoprevention, and treatment of colorectal cancer.” Expert review of clinical pharmacology vol. 13,10 (2020): 1125-1137.
7. Lisby, Amanda N., et al. “GUCY2C as a biomarker to target precision therapies for patients with colorectal cancer.” Expert Review of Precision Medicine and Drug Development 6.2 (2021): 117-129.
8. Shailubhai, K et al. “Uroguanylin treatment suppresses polyp formation in the Apc(Min/+) mouse and induces apoptosis in human colon adenocarcinoma cells via cyclic GMP.” Cancer research vol. 60,18 (2000): 5151-7 .
9. Maresca, Kevin P., et al. “Preclinical Evaluation of 89Zr-Df-IAB22M2C PET as an Imaging Biomarker for the Development of the GUCY2C-CD3 Bispecific PF-07062119 as a T Cell Engaging Therapy.” Molecular Imaging and Biology 23.6 (2021): 941-951.
10. Snook, Adam E., et al. “Split tolerance permits safe Ad5-GUCY2C-PADRE vaccine-induced T-cell responses in colon cancer patients.” Journal for immunotherapy of cancer 7.1 (2019): 1-12.